- Research Article
- Open access
- Published:
Scleractinian corals from the Lower Cretaceous of the Alpstein area (Anthozoa; Vitznau Marl; lower Valanginian) and a preliminary comparison with contemporaneous coral assemblages
Swiss Journal of Palaeontology volume 141, Article number: 3 (2022)
Abstract
From the Vitznau Marl (lower Valanginian) at the locality Wart in northeastern Switzerland (Alpstein area), 18 species from 17 genera and 13 families are described, including the genera Actinaraea, Actinastrea, Adelocoenia, Aplosmilia, Axosmilia, Complexastrea, Cyathophora, Dermosmilia, Fungiastraea, Heterocoenia, Latiastrea, Montlivaltia, Placophyllia, Pleurophyllia, Stylophyllopsis, Thamnoseris, and specimens showing affinities to solitary stylophyllids. The corals from the Vitznau Marl were derived from a limestone–marl alternation that is fossiliferous and clay-rich at the base (Vitznau Marl), containing crinoids, bryozoans, and sparse reworked corals and sponges. The coral fauna is distinctly dominated by forms belonging to the category of lowest to no polyp integration (50%), followed by species of the cerioid-plocoid group (33%) and forms having the highest polyp integration (thamnasterioid; 17%). With regard to polypar size, the Wart fauna is dominated by corals having large-size (> 9 mm) polyps (= 39%), followed by corals having medium- (> 2.5‒9 mm; 33%) and small-size polyps (up to 2.5 mm; 28%). Based on morphological features, the fauna from the Vitznau Marl closely corresponds to coral assemblages that are subjected to near-chronic, moderate sediment-turbidity stress that is punctuated by high-stress events, and that are largely or entirely heterotrophic. No coral fabric was observed that would suggest a biohermal development. But in a very small number of places, structures are present which might be fragments of crusts of microbialites, pointing to the hypothesis that at least a few of the corals might have been a part of some kind of bioconstruction. At the species-level, the fauna of the Vitznau Marl shows either no or very little affinities to other Valanginian assemblages such as to the fauna of Hungary (4.3%), followed by the associations of Ukraine, Switzerland (non-Vitznau), Spain (SpII), and Bulgaria. At the genus-level, the Wart fauna shows low correspondence to the fauna of Spain (SpII) (14.5%), followed by the assemblages of Hungary, Bulgaria, and Ukraine. In addition to the Vitznau Marl corals, an account of all Valanginian coral faunas published before early 2021 is given, including their paleogeographic distribution, as well as their taxonomic and morphological characterization. For this preliminary study, a total of 206 coral species belonging to 97 genera found in the coral assemblages of the Valanginian were included. At both the genus- and the species-levels, colonial taxa are most abundant (colonial genera: 89%; colonial species 90%). The vast majority of the Valanginian genera already occurred in older strata. Only 11 genera (out of 97 = 11%) are newly recorded. The Valanginian faunas having the largest number of solitary taxa lived in both (sub-) paratropical to warm-temperate areas, and in arid regions. The coral faunas of the Valanginian are distinctly dominated by corals of well-established microstructural groups. Only 13% of the species from 24% of the genera belong to “modern” groups. Compared to the situation in the Berriasian which showed that 9% of the species and 17% of the genera belonged to modern microstructural groups, the occurrence of “modern” groups significantly increased during the Valanginian.
Introduction
Lower Cretaceous scleractinian corals are known from a vast number of occurrences worldwide, especially for the period Hauterivian–middle Albian (e.g., Baron-Szabo, 1993, 1997, 2014, 2016, 2021a, 2021b; Baron-Szabo & Fernández-Mendiola, 1997; Baron-Szabo & González-León, 1999, 2003; Baron-Szabo & Steuber, 1996; Bover-Arnal, et al., 2012, Bugrova, 1990, 1999; Dietrich, 1926; Eguchi, 1951; Felix, 1891; Filkorn & Pantoja-Alor, 2009; Idakieva, 2007; Karakash, 1907; Koby, 1896, 1897, 1898; Kuzmicheva, 2002; Löser 2008; Löser, et al., 2015; Morycowa, 1964, 1971; Morycowa & Decrouez, 2006; Morycowa & Marcopoulou-Diacantoni, 2002; Morycowa & Masse, 1998; Pandey et al., 2007; Prinz, 1991; Reig Oriol, 1994; Reyeros de Castillo, 1983; Schöllhorn, 1998; Scholz, 1979, 1984; Shishlov, et al., 2020; Sikharulidze, 1977, 1979, 1985; Tomás et al., 2008; Toula, 1889; Turnšek, 1997; Turnšek & Buser, 1974, 1976; Turnšek & Mihajlović, 1981; Turnšek, et al., 1992; Von der Osten, 1957; Wells, 1932, 1933, 1944). For the lowermost Cretaceous, however, only a very small number of coral assemblages have been recorded, including European faunas of the Berriasian, e.g., southern Spain (Geyer & Rosendahl, 1985), Ukraine (Arkadiev & Bugrova, 1999; Kuzmicheva, 1972), western Austria and northeastern Switzerland (Baron-Szabo, 2018; Baron-Szabo & Furrer, 2018), as well as Asia (e.g., Tibet: Liao & Xia, 1994) and northern Africa (Beauvais & M’Rabet, 1977) (for discussions on Berriasian assemblages see Baron-Szabo, 2018). A small number of coral faunas from the Valanginian have been reported, including rare European and West Asian coral assemblages such as the ones from Bulgaria (Roniewicz, 2008), Hungary (Császár & Turnšek, 1996), and Ukraine (Kuzmicheva, 1967, 2002). Recently, the first works on the Lower Cretaceous corals from Argentina (Neuquén Basin) have been published, including material from the upper Valanginian (Agrio Formation) (Garberoglio et al., 2020, 2021). In addition, the first information on upper Valanginian material from northeastern Switzerland (Pygurus Member) has been reported from an ongoing study of the Pygurus coral fauna (Baron-Szabo & Furrer, 2018). While the results of these studies are yet to be finalized, they provide sufficient information to make some initial assessments. With regard to the latter study, the material found so far shows correspondence to some stylophyllid specimens of the assemblage of the Vitznau Marl. Regarding the Argentinian fauna, representatives of well-established microstructural groups such as actinastreids have been recorded (e.g., cf. Enallocoenia [referring to material assigned in Garberoglio, et al., 2020, to Stelidioseris gibbosa] and cf. Columactinastraea [referring to material assigned in Garberoglio, et al., 2021, to Eocolumastrea octaviae]).
With regard to Valanginian corals from Switzerland, only a very small number of occurrences have been known from central and western Switzerland (Haefeli, et al., 1965; Koby, 1896, 1897, 1898). Up to now, the only taxonomic descriptions and illustrations of Swiss scleractinian corals from this time period were published more than a century ago (Koby, 1896, 1897, 1898). Recently, as a part of a book project on fossils derived from the Cretaceous sediments in the Alpstein (Kürsteiner & Klug, 2018), the first Valanginian corals were collected from northeastern Switzerland. The purpose of this paper is to (i) taxonomically describe the first scleractinian coral assemblage from the lower Valanginian of the Vitznau Marl of northeastern Switzerland; (ii) provide both preliminary paleogeographic distributional patterns and paleoenvironmental occurrences, and (iii) provide a preliminary comparison of the coral fauna of the Vitznau Marl with contemporaneous scleractinian assemblages.
Materials, methods, abbreviations
Materials
In the current paper, 64 corals from the Vitznau Marl of northeastern Switzerland were identified by examining the corallum surface and using polished surfaces and thin sections. In general, the specimens are fragmented, their size ranging between several millimeters to around a decimeter. In most of the specimens, microstructural features are not preserved.
The material was collected by the coauthors Peter Kürsteiner (Nature Museum, St. Gallen), Karl Tschanz (Zurich) and Swiss collector Robin Näf (Wildhaus) during the years 2018 and 2020. Preparation of polished slabs and thin sections was carried out by Karl Tschanz. Material used in the current study is housed at the Nature Museum St. Gallen (inventory acronym of the Kürsteiner collection is NMSG Coll. PK). Further material mentioned in the current work is housed at the following institutions: MNHN: Museum National d’Histoire Naturelle, Paris, France; NMNHS: Natural Museum of Natural History, Sofia, Bulgaria; SNSB-BSPG: Bayerische Staatssammlung für Paläontologie und historische Geologie, Munich, Germany.
Methods
Identifications in the literature without descriptions or illustrations which have not been subsequently confirmed in taxonomic publications are excluded from taxonomic evaluation in the current work. Also excluded from a detailed comparison with other Valanginian faunas is a small number of works in which the stratigraphic ranges of the coral-bearing strata are not clearly defined but only given as, e.g., upper Berriasian–Valanginian, Valanginian–Hauterivian. These works refer to studies in the USA (Texas, Knowles Limestone; Scott, 1984; see stratigraphic update in the report by the Bureau of Ocean Energy Management [BOEM], 2012, p. 19 at: www.boem.gov); Armenia (Papoyan, 1982, 1989); Norway (Baron-Szabo, 2005); Poland (Kołodziej, 2003); South Africa (Kitchin, 1908); and Spain (Geyer & Rosendahl, 1985). However, in order to provide an idea about taxa which might eventually turn out to be of Valanginian age, a list of these coral occurrences is given in Appendix Table 5.
With regard to the size of corallites, the mean value is used when polypar size overlapped two categories.
Abbreviations
*=first description of taxon to which the assignment of specimen refers; v=material was studied by one of the authors (RBS); (v)=material not examined by author but considered to be sufficiently documented to be reliably identified; citation in italics in synonymy list= taxon only listed in the work concerned (neither illustration nor description provided).
Northeastern Switzerland (Cantons of Appenzell Innerrhoden, Appenzell Ausserrhoden, and St. Gallen) (Fig. 1):
-
Alpstein (mountain chain stretching over the Cantons of Appenzell Innerrhoden, Appenzell Ausserrhoden, and St. Gallen): 20–25 km south of St. Gallen.
-
Rotsteinpass (mountain pass in the Alpstein; Cantons of Appenzell Innerrhoden and St. Gallen): located about 2 km south-southeast of Säntis.
-
Säntis (highest mountain of the Alpstein; located about 18 km south of St. Gallen.
-
Wart (Alp in the Alpstein): located about 4 km southwest of Säntis.
Lithology and sedimentary environment of the Vitznau Marl
The Vitznau Marl is characterized by a succession of hemipelagic marl containing quartz-sand turbidites (Burger, 1985, 1986; Burger & Strasser, 1981; Föllmi, et al., 2007). It is underlain by a mixed sedimentation phase (Palfris Formation and the upper part of the Őhrli Formation [upper Őhrli Limestone]) and overlain by purely calcareous sediments of the Betlis Formation and Diphyoides Limestone (Fig. 2). In northeastern Switzerland, the Vitznau Marl is represented by a 130 m thick limestone–marl alternation that is fossiliferous and clay-rich in its lower section, containing crinoids and bryozoans. In addition, sparse reworked corals and sponges were found. These layers are followed by quartz-sand-rich and fossil-poor beds (Funk, 1999; Morales, et al., 2013; Sala, et al., 2014). In the Alpstein area, the Vitznau Marl crops out south of the area of the Rotsteinpass (Sala, et al., 2014) (Fig. 3). The Vitznau Marl represents deposits of the distal shelf in the open marine area of a platform along the northern margin of the Tethys (Jordi, 2012) (Fig. 4). Geographically, the Vitznau Marl stretches from western Austria (Vorarlberg), over eastern Switzerland to the Bernese Highlands in central-western Switzerland.
Time–space diagram of the sedimentary succession of the upper Berriasian to lower Valanginian in the Helvetic Alps. The space axis represents approximately 30 km in its totality (modified from Föllmi et al., 2007). Ls = limestone; Mb = member; Fm = formation
European Valanginian shorelines. Stipple indicates land areas; asterisk = area from which the corals of the Vitznau Marl were derived (modified from Tyson & Funnell, 1987). The paleocoordinates of the collecting site are: 38° 11’ N, 18° 10’ E (paleocoordinates estimated using information available on Paleobiology Database [paleobiodb.org])
The corals found in the Vitznau Marl are represented by fragmented coralla that were reworked and re-deposited. None of the specimens were found in situ. The coral material is embedded in a fine-grained matrix together with other mm- to cm-size bioclasts such as fragmented gastropods, serpulids, sponges, shell fragments, and others.
General attributes of the corals of the Vitznau Marl at Wart (Table 1)
Morphology
The coral material described in the current work comprises 64 specimens belonging to 18 species from 17 genera and 13 families. The fauna is distinctly dominated by forms belonging to the category of lowest to no polyp integration. Half of the fauna (50%) belong to this category (branching [6 species = 33%] and solitary [3 species = 17%]). This group is followed by species having types of polyp integration of the next higher integration arrangement (cerioid-plocoid group), to which a third of the coral species belong (6 species = 33%). The smallest group of colonial forms (3 species = 17%) consists of species having the highest polyp integration (thamnasterioid), including one showing mixed types of polyp integration combining high and lower integration arrangements (the cerio-thamnasterioid Thamnoseris cf. carpathica). The main morphotypes of non-branching corals from the Vitznau Marl are massive to subhemispherical. The branching species are phaceloid (Table 1).
Corallite size and polyp integration
With regard to polypar size, the Wart fauna is almost equally distributed by species with large-size (> 9 mm) polyps (7 species; 39%) and medium-size (> 2.5‒9 mm) polyped corals (6 species; 33%), followed by corals having small-size polyps (5 species = 28%). The group with large polyps is characterized by the category of corals having lowest to no polyp integration. All of the solitary corals (3 species: Axosmilia villersensis, Montlivaltia truncata, Stylophyllid indet. [questionably solitary]) and half of the branching types (3 species: Aplosmilia semisulcata, Dermosmilia sp., Placophyllia cf. florosa) belong to this group. The only species having a higher type of polyp integration in this group is Complexastrea zolleriana which belongs to the cerioid-plocoid category. No species having highly integrated polyps (thamnasterioid) has large polyps. It should be noted, however, that several representatives of the cerioid-plocoid species Complexastrea zolleriana found in the Vitznau Marl show growth forms that closely resemble the kinds of the lowest (branching) or no polyp integration, mimicking solitary, phaceloid, and subbranching-flabellate types. The medium-size group consists of branching (3 species: Placophyllia cf. dianthus, Pleurophyllia sp., Stylophyllopsis silingensis), cerioid-plocoid (2 species: Cyathophora claudiensis, Latiastrea mucronata), and thamnasterioid (1 species: Thamnoseris cf. carpathica) forms. Species having small-size corallites form the smallest group and belong to higher polyp integration categories, including 3 species of the cerioid-plocoid (Actinastrea pseudominima, Adelocoenia parvistella, Heterocoenia inflexa) and 2 species (Actinaraea tenuis, Fungiastraea lamellosa) of the thamnasterioid groups. Neither solitary nor branching species were found in the group having small-size polyps (Table 1).
Paleoecology of the corals of the Vitznau Marl
Among other skeletal features, the corallite size found in corals has been used for paleoenvironmental interpretations (Baron-Szabo, 2021a, 2021b; Baron-Szabo & Sanders, 2020; Dimitrijević, et al., 2020; Sanders & Baron-Szabo, 2005 [and references therein]; Santodomingo, 2014). In the fauna of the Vitznau Marl, corallite size of the corals varies from less than 1 mm to more than 20 mm but smaller corallites are less common. Forms having medium- to large-size corallites are most abundant (13 species = 72%). Only 5 species (28%) belong to the small-polyp group. Furthermore, Wart corals belonging to the category consisting of forms having lowest to no polyp integration (solitary and branching types) form the most dominant group (50%), followed by species having types of polyp integration of the next higher integration arrangement (cerioid-plocoid group; 33%). Species having the highest polyp integration (thamnasterioid; 3 species = 17%) form the smallest group. Interestingly, several species belonging to the medium- and high-polyp integration groups show pseudo-branching morphotypes: (1) some specimens belonging to Complexastrea zolleriana (= cerioid-plocoid, large-polyp category) have growth forms closely resembling branching to uniserially flabellate types as are typical of genera such as Thecosmilia (= branching-phaceloid) and Latiphyllia (= subbranching to flabellate, generally uniserial); and (2) some specimens belonging to the cerioid-plocoid (small- to medium-polyps; Actinastrea and Latiastrea) and thamnasterioid (Actinaraea) categories are ramose to subcolumnar. Recent zooxanthellate coral assemblages are characterized by high-integrated species predominantly having smaller corallite diameters (1–5 mm) (Coates & Jackson, 1987; Stafford-Smith, 1993), thus significantly differing from the Wart fauna. According to, e.g., Bak and Elgershuizen (1976), Logan (1988), and Stafford-Smith (1993), corals having large-size corallites effectively reject sediment up to fine gravel-size, whereas small-polyped corals can be effective in rejection of clay to silt. In the fossil record, coral faunas characterized by species with small polyps have been reported from, e.g., the marls of the Upper Cretaceous Gosau Group (e.g., Baron-Szabo, 1997, 2003, 2014; Beauvais, 1982), from marly sediments of the Maastrichtian of Jamaica (Baron-Szabo, 2008), and from the marly to sandy-marly strata of the Oligocene of Italy (Crosara-horizon; Pfister, 1980). Coral assemblages characterized by large-size corallites rejecting sediment up to fine gravel-size have been described from the Miocene of the Caribbean (Montebello Member, Lares Formation; Champagne, 2010). From that it can be said, as a trend, that the corals of the Vitznau Marl might have been predominantly affected by coarse, up to gravel-size sediment. Considering that (1) solitary forms together with half the number of the low-integrated taxa (branching types) form the largest group; (2) even corals belonging to taxa of higher types of polyp integration developed pseudo-branching growth forms (e.g., Complexastrea zolleriana); and (3) some specimens of the cerioid-plocoid Complexastrea zolleriana that correspond to the “Coenotheca-stage” show close resemblance to mobile forms which are known for their high resilience to ecostress (e.g., Manicina areolata (Linnaeus, 1758) (Ginsburg, 1972; cf. Sanders & Baron-Szabo, 2005), the Wart fauna closely corresponds to coral assemblages that are subjected to near-chronic, moderate sediment-turbidity stress that is punctuated by high-stress events, and that are largely or entirely heterotrophic (Dryer & Logan, 1978; Sanders & Baron-Szabo, 2005). Generally, no coral fabric was observed that would suggest a biohermal development but in a very small number of places, structures are present which might be fragments of crusts of microbialites, pointing to the hypothesis that at least some corals of the Vitznau Marl might have formed some kind of bioconstruction. This implies, as a hypothesis, that the Wart fauna might have been a non-reefal, azooxanthellate community with some indication that at least a small number of corals were involved in early biohermal developments (“pioneer stage”). This closely corresponds to earlier findings showing the great range of tolerance of most of the Wart taxa (Table 1). Coral assemblages that are comparable to the Swiss fauna have been reported from various time periods such as the middle Jurassic of east-central Iran (Baghamshah Formation, Bathonian‒middle Callovian; Pandey & Fürsich, 2003) and the uppermost Lower Cretaceous of the USA (Smeltertown Formation, Albian; Turnšek, et al., 2003). At the Iranian locality, coral associations were reported from marly-silty to strongly silty-sandy sediments in mainly non-reefal and (one) biohermal development (patch-reef). The corals from the Albian Smeltertown Formation were derived from a sedimentary succession that gradually shallows from offshore marine conditions to a hyposaline lagoonal paleoenvironment with sedimentation shifting from dark-grey shale to clastic turbiditic material and resedimented carbonates. The corals were found at the base of a non-reefal shallowing-upward cycle.
Distribution of the corals of the Vitznau Marl
The corals of the Vitznau Marl are dominated by forms that are restricted to the Lower Cretaceous (9 taxa = 50%), followed by species that had their first occurrence in the Jurassic (7 taxa = 39%). Only 2 species (11%) have been known from Lower and Upper Cretaceous strata, both of which belong to the category of high-polyp integration (thamnasterioid group) (Actinaraea tenuis, Thamnoseris carpathica). No coral species of the new material occurred in strata that are both older and younger than the Lower Cretaceous (Table 2).
At the species-level, when compared to the six largest Valanginian faunas recorded, the fauna of the Vitznau Marl shows no or very little affinities to any of them (Hungary, followed by the associations of Ukraine, Switzerland [non-Vitznau Marl], of southern Spain [SpII; lower Valanginian], and Bulgaria [all between 1.3‒4.3%]). None of the species are shared with the upper Valanginian fauna of southern Spain (SpI) (Table 3; Appendix Table 8).
At the genus-level, the fauna of the Vitznau Marl shows correspondence to all six Valanginian faunas that are used for more detailed comparison at very low to low ranges. The closest correspondence is to the fauna of Spain (SpII; 14.5%), followed by the assemblages of Hungary, Bulgaria, Ukraine, Switzerland (non-Vitznau Marl), and southern Spain (SpI) (all between 3.5‒11.5%). Only two genera are shared with the fauna of Switzerland (non-Vitznau Marl), both of which are solitary forms (Axosmilia, Montlivaltia) (Table 4).
Valanginian coral assemblages worldwide
Though scleractinian coral assemblages of the Valanginian have been reported from a rather small number of localities (probably less than two dozen), they, however, have been known worldwide (Fig. 5). In the current work, in addition to the Wart fauna, twelve Valanginian coral faunas are reviewed, six of which are compared in more detail with the coral fauna of the Vitznau Marl: Ukraine (Kuzmicheva, 1967, 1985, 2002); western-central Switzerland (non-Vitznau Marl; Fromentel, in Fischer, 1873; Haefeli, et al., 1965; Jaccard, 1893); southern Spain (two faunas [Sp (I) and Sp (II)]; Löser et al., 2019, Löser et al., 2021); Hungary (Császár & Turnšek, 1996); and Bulgaria (Roniewicz, 2008).
Map showing localities of Valanginian coral faunas included in this study. X = Wart (Vitznau Marl) corals (current paper), 18 species; 1 = Mexico (Sandy, 1990; Wells, 1946), 3 species; 2 = Spain [II] (Löser, et al., 2021), 42 species; 3 = Spain [I] (Löser, et al., 2019), 19 species; 4 = France (Masse, et al., 2009), 2 species; 5 = Switzerland (non-Vitznau Marl) (de Fromentel, in Loriol, 1868; Haefeli, et al., 1965; Jaccard, 1893; Koby, 1896, 1897, 1898), 21 species; 6 = Slovenia (Turnšek, 1997; Turnšek & Buser, 1974), 3 species; 7 = Hungary (Császár & Turnšek, 1996), 29 species; 8 = Bulgaria (Roniewicz, 2008), 62 species; 9 = Poland (Lefeld, 1968), 1 species; 10 = Turkey (Kaya et al., 1987), 2 species; 11 = Ukraine (Kuzmicheva, 1967, 1985, 2002), 18 species; 12 = Tanzania (Dietrich, 1926; Löser, 2008), 7 species (for coordinates and paleocoordinates see caption of Fig. 6)
The number of species of each of these assemblages ranges from 18 (Ukraine) to 62 (Bulgaria) (Table 3). Taking into consideration the overall low numbers of both the Valanginian coral assemblages and coral taxa, as a trend it can be said as a preliminary conclusion that:
-
a total of 206 coral species belonging to 97 genera were found in the coral assemblages of the Valanginian (Appendix Tables 7 and 8);
-
at both the genus- and the species-levels, colonial taxa are most abundant during the Valanginian (colonial genera: 89%; colonial species 90%);
-
the vast majority of the Valanginian genera already occurred in older strata. Only 11 genera (out of 97 = 11%) are newly recorded, eight of which are endemic during the Valanginian (marked by *; also see Appendix Table 7): Agathelia*, Confusaforma*, Eugyra*, Lyubasha*, Microsolenastraea*, Palaeopsammia*, Paretallonia, Siderastreites*, Siderofungia*, Thalamocaeniopsis, and Tricassastraea (Lathuilière, 1989; Paleobiology Database at paleobiodb.org; and updated herein);
-
based on the succession of microstructural groups, Roniewicz and Morycowa (1993) distinguished four stages in the history of Scleractinia: I. Early Mesozoic stage; II. Middle Mesozoic stage; III. Late Mesozoic stage; and IV. Cenozoic stage. In this model, the Valanginian period belongs to phase 2 of the Middle Mesozoic stage (Oxfordian–Valanginian). This stage is characterized by (1) the continued presence of earlier microstructural types (types having rather thick trabeculae, such as haplaraeids, comoseriids, latomeandrids, and thecosmiliids; and small-to-medium size trabeculae forms, such as the actinastreids); (2) the diversification of auricular (stylinid) and amphiastreid corals; and (3) the appearance of new microstructural types such as minitrabeculae (as in Myriophyllia), as well as types forming densely branching trabeculae (as seen in neo-rhipidicanth and rhipidogyrid taxa), and groups having thick and horizontally (= parallel to corallum base) arranged trabeculae (heterocoeniids). According to Roniewicz and Morycowa (1993), a sharp limitation of microstructural development of all but newly established coral groups occurred after the Tithonian. From that it can be concluded that the scleractinian coral faunas of the Valanginian are distinctly dominated by corals of well-established microstructural groups: only 27 species (out of 206 = 13%) from 23 genera (out of 97 = 24%) belong to “modern” groups (Appendix Tables 7 and 8);
-
in a recent work on the worldwide occurrence of Berriasian corals (Baron-Szabo, 2018), 113 species belonging to 57 genera were identified. It was found that 10 species (9%) from 10 genera (18%) were representatives of modern microstructural groups. From that it can be said that the number of “modern” taxa significantly increased in the Valanginian by 33% (genus-level) and 44% (species-level), respectively;
-
all of the most-widespread genera during the Valanginian (= reported from three or more localities) belong to well-established microstructural groups (Actinaraea*, Actinastrea*, Adelocoenia*, Ahrdorffia, Axosmilia*, Cladophyllia*, Comoseris*, Cyathophora*, Dimorphastrea*, Ellipsocoenia*, Heliocoenia*, Latiastrea*, Microphyllia, Microsolena, Montlivaltia*, Periseris*, Placophyllia*, Stylina, Stylosmilia*, Synastrea, Tricassastraea) (Appendix Table 7), 15 of which (marked by *) were reported from a wide latitudinal range, including tropical to warm-temperate regions. The genera Actinastrea, Dimorphastrea, and Montlivaltia were additionally reported from arid areas (Fig. 6);
Fig. 6 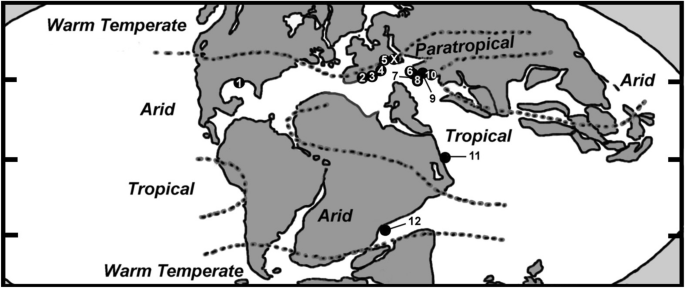
modified from Paleomap project Scotese [2014] at www.scotese.com; and Tennant, et al., 2017). Paleocoordinates of the coral localities of Spain [I, II] and Vitznau Marl at Wart estimated based on information provided by Paleobiology Database (paleobiodb.org); all others retrieved from Paleobiology Database (paleobiodb.org)
Simplified Lower Cretaceous paleogeographic map showing the occurrences of species during the Valanginian: X = Wart (Vitznau Marl) corals (current paper); 1 = Mexico (Sandy, 1990; Wells, 1946); 2 = Spain [II] (Löser, et al., 2021); 3 = Spain [I] (Löser, et al., 2019); 4 = France (Masse, et al., 2009); 5 = Switzerland (non-Vitznau Marl) (de Fromentel, in Loriol, 1868; Haefeli, et al., 1965; Jaccard, 1893; Koby, 1896, 1897, 1898); 6 = Slovenia (Turnšek, 1997; Turnšek & Buser, 1974); 7 = Hungary (Császár & Turnšek, 1996); 8 = Bulgaria (Roniewicz, 2008); 9 = Poland (Lefeld, 1968); 10 = Turkey (Kaya, et al., 1987); 11 = Ukraine (Kuzmicheva, 1967, 1985, 2002); 12 = Tanzania (Dietrich, 1926; Löser, 2008). Note that the coordinates (and paleocoordinates) of the Valanginian localities are: 1 = 25º 30 ‘ N, 103º 30 ‘W (23º 6 ‘N, 56º 30 ‘ W); 2 = 38º N, 3º W (29º N, 2º E); 3 = 38º N, 1º W (29º N, 7º E); 4 = 43° 12’ N, 5° 24’E (34° 12’ N, 14° 30 E); 5 = 47º N, 7º E (28º N, 16º E); 6 = 46° N, 13° 42’ E (31° 6’ N, 21° 6’ E); 7 = 46° 12’ N, 18° 18’ E (28° 24’ N, 24° 42’ E); 8 = 42° 48’ N, 22° 48’ E (24° 0’ N, 25° 24’ E); 9 = 50° 0’ N, 19° 48’ E (31° 0’ N, 28° 18’ E); 10 = 40° 48’ N, 29° 24’ E (36° 36’ N, 36° 54’ E); 11 = 44° 48’ N, 34° 30 E (0° 30’ S, 49° 30 E); 12 = 9° 42’ S, 39° 18’ E (31° 48’ S, 18° 48 E); X = 47° 14′ 36″ N, 9° 21′ 55″ E (38° 11’ N, 18° 10’ E). Paleomap
-
the occurrence of taxa belonging to modern microstructural groups is nearly exclusively restricted to tropical–subtropical (= assemblages of Bulgaria, Hungary, Slovenia, Spain [I], Spain [II], Ukraine) and arid regions (Mexico). Only two modern-group taxa (the branching form Aplosmilia semisulcata and the cerio-plocoid Heterocoenia inflexa) were found in a (sub-) paratropical to warm-temperate region (Wart fauna, Vitznau Marl) (Appendix Tables 7 and 8);
-
at the genus-level, taxa belonging to modern microstructural groups were nearly or completely isolated geographically: representatives of Starostinia only occurred in the fauna of Slovenia, forms of Columnocoenia and Diplocoenia occurred in the assemblages of both Hungary and Ukraine, and the faunas of Spain (SpI) and Mexico contain species of Paretallonia. All of their respective species are endemic during the Valanginian (Appendix Table 8);
-
at the species-level, all of the forms which occurred in more than one locality during the Valanginian belong to well-established microstructural groups (Actinaraea tenuis, Ahrdorffia ornata, Axosmilia villersensis, Comoseris jireceki, Cyathophora claudiensis, Cyathophora hexalobata, Dimorphastrea explanata, Ellipsocoenia haimei, Latiastrea mucronata, Periseris lorioli) (Appendix Tables 7 and 8);
-
the Valanginian faunas having the largest numbers of solitary taxa lived in both (sub-) paratropical to warm-temperate areas (Switzerland [coral faunas of Wart [Vitznau Marl] and non-Vitznau Marl]; France) and in arid regions (Mexico, Tanzania) (paleogeography, paleoclimate, and paleoceanography data retrieved from Tennant, et al., 2017 and Paleomap project at www.scotese.com);
-
with regard to the six Valanginian faunas used for more detailed comparison, they either completely consist of or are dominated by colonial coral genera/species, ranging from 67%/76% (western-central Switzerland [non-Vitznau Marl; SII]) to 100% (Spain [SpI]) (Appendix Table 6);
-
at the species-level, the six Valanginian coral faunas from which the largest number of corals were recorded are distinctly dominated by taxa that are endemic in the Valanginian (Table 3; Appendix Tables 7 and 8), which is in great contrast to the distributional patterns of both the Upper Jurassic and most of the post-Valanginian Lower Cretaceous faunas (Hauterivian–Middle Albian). While many Jurassic and post-Valanginian assemblages are characterized by cosmopolitan to subcosmopolitan taxa (for the Jurassic, see, e.g., Beauvais, 1973, 1989; Errenst, 1990, 1991; Lathuilière, et al., 2020; Morycowa, 2012; Roniewicz, 1976; Turnšek, 1997; for the Lower Cretaceous, see e.g., Baron-Szabo, 2014, Baron-Szabo, 2021b; Baron-Szabo & González-León, 2003; Morycowa, 1971; Morycowa & Masse, 1998, 2009; Morycowa & Marcopoulou-Diacantoni, 2002; Pandey et al., 2007; Turnšek, 1997), the Valanginian faunas appear largely isolated (Table 3).
Systematic paleontology
The taxonomic framework applied here is based on the works by Milne Edwards and Haime (1857), Duncan (1884), Vaughan and Wells (1943), Alloiteau (1952, 1957), Wells (1956), Eliášová (1990), Baron-Szabo (2014, 2021c) for higher-level taxa (family), with updates on individual genera and species by Baron-Szabo (2014, 2021b) and Morycowa and Roniewicz (2016). Because the suborders of Vaughan and Wells (1943) are neither monophyletic nor do they conform with recent molecular results, they are no longer useful and are, therefore, excluded from the taxonomic framework. Because the taxonomy of scleractinian corals is in a transitional phase (Scleractinian Treatise is in progress; an increasing number of contradicting taxonomic approaches have been applied [see discussion in Kołodziej & Marian, 2021]; etc.), in the current work, the taxonomic framework is based on levels no higher than family (excluding superfamily and higher). For further information on excluded taxonomic frameworks of scleractinian corals see Baron-Szabo (2021b).
Family Actinastreidae Alloiteau, 1952
Remarks.
In some recent publications (e.g., Garberoglio, et al., 2020; Löser, 2012), material was grouped with the family Actinastreidae that showed characteristics of families such as Columastreidae, Cladophylliidae, and others (e.g., Baron-Szabo, 2014, p. 20; and discussion in Baron-Szabo, 2021b under Cladophyllia crenata). Therefore, a combination of the family concepts by Alloiteau (1954) and Baron-Szabo (2014) is followed.
Genus Actinastrea d’Orbigny, 1849
Type species. Actinastrea goldfussi d’Orbigny, 1850a, 1850b, Maastrichtian of The Netherlands (Maastricht).
Diagnosis. Corallum colonial, often massive to subcolumnar, cerioid, cerio-plocoid. Budding extracalicular and extracalicular-marginal. Corallites small and prismatic in outline, often directly united by their walls. Columella styliform or short-lamellar. No intercalicinal coenosteum. Synapticular structures present peripherally. Paliform structures occasionally present. Endothecal dissepiments thin, sometimes arranged forming an inner-corallite ring which is complete or incomplete. Septa compact, generally non-confluent, radially or bilaterally arranged, beaded marginally, and composed of a series of simple trabeculae, varying in diameter (up to 150 µm). Septal flanks covered by spiniform granulae. Wall septothecal to septoparathecal, with occasionally occurring pores (lacunes).
Actinastrea pseudominima (Koby, 1897 )
Fig. 7A
A Actinastrea pseudominima (Koby, 1897), NMSG Coll. PK 02.10.33c; calicular view of colony, thin section; scale bar: 1.5 mm. B Complexastrea zolleriana (Quenstedt, 1879), NMSG Coll. PK 02.10.20a; calicular view of colony in Latiphyllia-type stage, thin section; scale bar: 3.5 mm. C Complexastrea zolleriana (Quenstedt, 1879), NMSG Coll. PK 02.10.20a; upper surface of colony, lateral view; scale bar: 5 mm. D Complexastrea zolleriana (Quenstedt, 1879), NMSG Coll. PK 02.10.20b; calicular view of colony in Coenotheca–Complexastrea-type stage, thin section; scale bar: 4 mm. E Complexastrea zolleriana (Quenstedt, 1879), NMSG Coll. PK 02.10.36; calicular view of colony in Montlivaltia–Coenotheca-type stage, polished surface; scale bar: 5 mm. F Complexastrea zolleriana (Quenstedt, 1879), NMSG Coll. PK 02.10.36; calicular view of colony in Montlivaltia–Coenotheca-type stage, polished slab; scale bar: 5 mm. G Montlivaltia truncata (Defrance, 1817), NMSG Coll. PK 02.10.25a; calicular view, polished; scale bar: 3 mm. H Montlivaltia truncata (Defrance, 1817), NMSG Coll. PK 02.10.25a-I; calicular view, polished, slightly oblique; scale bar: 6.5 mm. I Montlivaltia truncata (Defrance, 1817), NMSG Coll. PK 02.10.22d; polished surface of oblique lateral-calicular view, polished; scale bar: 5 mm. J Montlivaltia truncata (Defrance, 1817), NMSG Coll. PK 02.10.25a (= 25a-I); upper surface of corallum, lateral view, slightly oblique; scale bar: 5 mm
-
v*1897 Astrocoenia pseudominima, Koby, 1896: Koby, p. 59, Pl. 15, Figs. 4–4a [topotypes studied].
-
nonv1926 Astrocoenia pseudominima Koby: Dietrich, p. 93, Pl. 6, Fig. 9.
-
v1936 Astrocoenia pseudominima Koby, 1896: Hackemesser, 1936, p. 71, Pl. 7, Fig. 14.
-
non1961 Actinastraea cf. pseudominima Koby: Bendukidze, p. 8.
-
v1964 Actinastraea pseudominima (Koby, 1896): Morycowa, p. 18–20, Pl. 1, Fig. 2–5, Pl. 2, Fig. 2.
-
v1971 Actinastraea pseudominima pseudominima (Koby, 1896): Morycowa, p. 33–37, Pl. 1, Fig. 1–2, Pl. 3, Fig. 1, Pl. 4, Fig. 1, Pl. 5, Fig. 3, Text-Figs. 6A, 11–12.
-
1977 Actinastrea cf. pseudominima (Koby, 1896): Sikharulidze, p. 69–71, Pl. 7, Fig. 1.
-
non1985 Actinastrea pseudominima (Koby, 1897): Geyer & Rosendahl, p. 167, Pl. 2, Fig. 1.
-
1988 Actinastraea pseudominima (Koby, 1896): Kuzmicheva & Aliev, p. 154, Pl. 1, Fig. 1a–b.
-
nonv1989 Actinastrea cf. pseudominima (Koby, 1896): Löser, p. 98–99, Pl. 21, Fig. 1, Text-Fig. 3.
-
nonv1992b Actinastraea pseudominima (Koby, 1896): Eliášová, 1992b, p. 402, Pl. 5, Fig. 3.
-
v1995 Actinastraea pseudominima (Koby, 1896): Abdel-Gawad & Gameil, 1995, p. 8, Pl. 4, Figs. 3–6.
-
1998 Actinastrea aff. pseudominima (Koby, 1897): Morycowa & Masse, p. 738, Fig. 10.2.
-
v2003 Actinastrea aff. pseudominima (Koby, 1897): Baron-Szabo, et al., 2003 p. 201, Pl. 36, Figs. 5–6.
-
2009 Actinastrea pseudominima (Koby, 1897): Filkorn & Pantoja-Alor, p. 23–25, Figs. 9.1–8 (older synonyms cited therein).
-
v2018 Actinastrea pseudominima (Koby, 1897): Baron-Szabo, p. 30, Pl. 1, Fig. A.
-
v2021b Actinastrea pseudominima (Koby, 1897): Baron-Szabo, p. 31, Pl. 1, Fig. C–D.
Dimensions of skeletal elements. Diameter of corallites: 1–2.2 mm, in areas of intense budding it can be 0.8 mm; distance of corallite centers: 1.1–2.7 mm; septa/corallite: 24, in corallites in areas of intense budding: 12.
Description. Small massive to subramose, cerioid to cerio-plocoid colony with often prismatic corallites. Costosepta arranged in 3 complete cycles in 6 systems in largest corallites. Columella styliform or short-lamellar. Endothecal dissepiments thin. Wall paraseptothecal to septothecal.
Type locality of species. Aptian of Switzerland (Reignier).
Distribution. Upper Berriasian (upper Őhrli Formation; Rotsteinpass, Lisengrat, Alpstein area) and lower Valanginian of northeastern Switzerland (Vitznau Marl, Wart, this paper), lower Barremian of Georgia (in Caucasia) and Turkmenistan, Barremian of Azerbaijan and Poland, upper Barremian–lower Aptian of Switzerland (Schrattenkalk Formation, Tierwis area) and France, lower Aptian of Mexico and Romania, upper Aptian of Iran, Aptian of Switzerland (Reignier), Albian of Egypt.
Material. NMSG Coll. PK–02.10.33c.
Remarks.
In having 10–12 septa, the material described as A. cf. pseudominima from the Hauterivian of Crimea in Bendukidze (1961) differs from Koby’s species. In having corallite diameters that are significantly larger than 2 mm, the material described in Dietrich (1926, p. 93) from the Lower Cretaceous of Tanzania differs from the species A. pseudominima. The specimens described from the upper Cenomanian of Germany (Löser, 1989, p. 98–99) and from the upper Cenomanian–lower Turonian of the Czech Republic (Eliášová, 1992b, p. 402) are more closely related to A. tourtiensis (Bölsche, 1871). For additional synonyms of the species A. pseudominima, see Paleobiology Database (paleobiodb.org).
Family Thecosmiliidae Duncan, 1884
Genus Complexastrea d’Orbigny, 1850b
Type species. Complexastrea subburgundiae d’Orbigny, 1850b, Jurassic (‘Corallien’) of France.
Diagnosis. Colonial, often massive to subhemispherical, subbranching to subflabellate during various stages of astogeny, present or absent. Polyp integration astreoid, plocoid to cerio-plocoid; submeandroid to thamnasterioid integration during various stages of astogeny present or absent. Budding intracalicular and extracalicular. Costosepta compact, non-confluent to confluent, granulated and carinate laterally. Their axial ends can be rhopaloid. Columella absent or formed by weakly parietal structures. No pali. Synapticulae absent. Intertrabecular distance ranging between 200 and 1300 µm. Endothecal dissepiments pass from one corallite to the next, they are vesicular, cellular, or tabuloid. Wall absent or paraseptothecal.
Complexastrea zolleriana (Quenstedt, 1879 )
Figs. 7B–F
-
*1879 Coenotheca zolleriana: Quenstedt, p. 609, Pl. 165 figs. 36-43.
-
(v)1996 Complexastrea zolleriana (Quenstedt): Lathuilière, p. 597, Pl. 72, Fig. 1–18, Pl. 73, Fig. 1–13, Pl. 74, Fig. 1–4, Pl. 75, Fig. 1–7, Text-Figs. 5–6, 8–11 [older synonyms cited therein].
-
pars(v)2003 Montlivaltia decipiens (Goldfuss 1826): Pandey & Fürsich, p. 42–43, Pl. 10, Fig. 7–10, [?Pl. 8, Fig. 6].
-
(v)2003 Latiphyllia cf. confluens (Quenstedt, 1843): Pandey & Fürsich, p. 44–46, Pl. 11, Fig. 1.
-
(v)2003 Coenotheca zolleriana Quenstedt, 1881: Pandey & Fürsich, p. 46–48, Pl. 11, Fig. 2–6.
Dimensions. Great diameter of corallites: 10–30 mm; in areas of intense budding around 8 mm; septa/corallite: up to around 100, in corallites in areas of intense budding around 20; septa/mm: 3–9/5; distance of corallite centers: 5–21 mm; septal thickness ranges between 100 and 1300 µm.
Description. Individual specimens are in various transgeneric stages including (1) cerio-plocoid to astreoid corallites in subflabellate arrangement (resembling Latiphyllia); (2) small submassive clumps consisting of a few polyps in subplocoid to submeandroid-thamnasterioid arrangement (resembling Coenotheca); and (3) tall corallum with subplocoid corallites (resembling Thecosmilia); costosepta straight to very wavy; endothecal dissepiments numerous, large vesicular peripherally, cellular to vesicular in central areas of corallum.
Type locality of species. Middle Jurassic (“Brauner Jura gamma”) of Germany (Hohenzollern).
Distribution. Middle Jurassic of France, Germany, and Iran, lower Valanginian of northeastern Switzerland (Vitznau Marl, Wart; this paper).
Material. NMSG Coll. PK–02.10.20a, 20b (= 02.10.21a), and 20c; –02.10.21c; –02.10.25a-II; –02.10.27c-A. –02.10.27c-B; –02.10.27d; –02.10.36; ?–02.10.38.
Remarks.
Based on a population study including Middle Jurassic thecosmiliid corals, Lathuilière (1996) established a morphogenesis framework for the genus Complexastrea using individuals that, with regard to the dimensions of their skeletal elements, correspond to the species zolleriana. He came to the conclusion that during various stages of astogeny forms of the genus Complexastrea show morphological overlaps with several other thecosmiliid genera such as Coenotheca, Latiphyllia, Montlivaltia, and Thecosmilia. As a result, specimens that might show close resemblance to one of these genera are grouped with Complexastrea. While this approach has not been accepted by some authors (e.g., Pandey & Fürsich, 2003), it is followed here based on the fact that the genus Complexastrea shows features that clearly distinguishes it from the genera with which it shows morphological convergence during different stages of astogeny. In having both extracalicular budding in addition to intracalicular multiplication and a paraseptothecal wall, Complexastrea differs from genera such as Latiphyllia and Thecosmilia (= characterized by intracalicular multiplication and the lack of septothecal thickenings). In its early (“solitary”) stage, Complexastrea closely resembles the solitary genus Montlivaltia but shows features such as (1) cerio-plocoid shapes and often paraseptothecal developments in the peripheral areas; (2) a rather flat to cupolate corallum; and (3) the presence of budding spots (“generator septa” and “linking septa” sensu Lathuilière [1996, Text-Fig. 10A‒B]) from which new corallites develop, closely corresponding to the situation in other genera such as the cunnolitid genus Aspidastraea which can be misinterpreted for the solitary genus Cunnolites in its early stages of astogeny (Baron-Szabo, 2003). In contrast to the “Montlivaltia”- stage of Complexastrea, the genus Montlivaltia is variably conical (rarely subdiscoidal-patellate in certain environments), and lacks both septothecal thickenings and budding spots. The genus Coenotheca very closely corresponds to the juvenile stage of Complexastrea and is, therefore, considered to be a junior synonym of Complexastrea.
Individual specimens from the Vitznau Marl correspond to different morphotypes of the species C. zolleriana. The specimen NMSG Coll. PK-02.10.20a resembles the “Latiphyllia” variation as shown in Lathuilière (1996, Pl. 72, Figs. 8 and 15, and Text-Fig. 10A–B); the specimens NMSG Coll. PK-02.10.20c, 02.10.25a-II, and 02.10.36 show close affinities to the “Coenotheca” morphotype as shown in Lathuilière (1996, Pl. 72, Figs. 7 and 10); the specimen NMSG Coll. PK-02.10.20b (= 02.10.21a) resembles the “Thecosmilia” variation as shown in Lathuilière (1996, Pl. 72, Fig. 5); the specimen NMSG Coll. PK-02.10.21c corresponds to a mix of transgeneric stages including variation of “Complexastrea–Coenotheca–Thecosmilia” as shown in Lathuilière (1996, Pl. 73, Fig. 5–8); and the specimens NMSG Coll. PK-02.10.27c-A and B, and –02.10.27d show close affinities to early stages of astogeny of Complexastrea, corresponding to a mix of “Coenotheca–Montlivaltia” morphotypes as shown in Lathuilière (1996, Pl. 72, Figs. 3 and 6).
Genus Montlivaltia Lamouroux, 1821
Type species. Montlivaltia caryophyllata Lamouroux, 1821, Middle Jurassic (Upper Bathonian) of Calvados.
Diagnosis. Solitary, trochoid to subcylindrical, or turbinate, rarely (subdiscoidal-) patellate. Costosepta compact, thin to thick, exsert, in general numerous and crowded. Columella absent. Intertrabecular distance ranging between 200 and 1300 µm. Endothecal dissepiments abundant, vesicular. Epitheca sensu lato membraniform or absent.
Montlivaltia truncata (Defrance, 1817 )
Figs. 7G–J
-
*1817 Caryophyllia truncata: Defrance, vol. 7, p. 198.
-
v1954 Montlivaltia truncata (Defrance) 1817: Geyer, 1954, p. 174 [older synonyms cited therein].
-
(v)1977 Montlivaltia truncata Defrance, 1817: Beauvais, in Beauvais & M’Rabet, p. 109, Pl. 1, Fig. 3a–b, Pl. 2, Fig. 2 [older synonyms cited therein].
-
(v)1977 Montlivaltia sioufensis nov. sp.: Beauvais, in Beauvais & M’Rabet, p. 112, Pl. 3, Fig. 1.
-
(v)1994 Montlivaltia xizangensis Liao et Xia: Liao & Xia, p. 158, Pl. 43, Figs. 9–14 and 20–23.
-
v2018 Montlivaltia truncata (Defrance, 1817): Baron-Szabo, p. 38–39, Pl. 2, Figs. I–J [older synonyms cited therein].
Dimensions. Corallite diameter at top of corallum (d x D): 12 × 18 mm (estimated); d/D = 0.67; diameter at basal part of corallum (d x D): 10 × 12 mm (estimated); d/D = 0.83; septa at top of corallum; probably 96; septa at basal part: around 40; septa/mm (peripheral area of corallite): 7–10/5; height of corallum: at least 35 mm.
Description. Incomplete solitary, turbinate, corallum; septa straight, regularly alternate in length and thickness, probably developed in 5 complete cycles in 6 systems at top of corallum; endothecal dissepiments numerous, long, mainly vesicular; membraniform epitheca sensu lato present.
Type locality of species. Upper Jurassic of France.
Distribution. Upper Jurassic of France, Germany, and Switzerland, upper Oxfordian of Azerbaijan and Georgia (in Caucasus), upper Oxfordian–lower Tithonian of Russia, Kimmeridgian of Portugal, Berriasian of central Tibet, upper Berriasian of northern Tunisia and northeastern Switzerland (upper Őhrli Formation), lower Valanginian of northeastern Switzerland (Vitznau Marl, Wart; this paper).
Material. NMSG Coll. PK–02.10.25a (= 02.10.22d).
Remarks.
Because the specimen is incomplete the full range of its dimensions of skeletal elements can only be estimated. The dimensions found in the Swiss material closely correspond to the ones of M. truncata.
The species M. truncata was recently discussed and revised (Baron-Szabo, 2018). For further synonyms of the species M. truncata, see Paleobiology Database (paleobiodb.org).
Family Placophylliidae Eliášová, 1990
Genus Placophyllia d’Orbigny, 1849
Type species. Lithodendron dianthus Goldfuss, 1827, Upper Jurassic of Germany (Giengen).
Diagnosis. Colonial, mainly phaceloid, can be subdendroid or fasciculate with plocoid to cerioid polyp outlines in younger colonies. Budding extracalicular and intracalicular-marginal. Costosepta compact, septal flanks covered by small granules. Distal edge of septa smooth. In closely packed corallites costae may be subconfluent. Columella lamellar. Synapticulae and pali absent. Intertrabecular distance ranging between 40 and 130 µm in septa. Corallite wall parathecal, irregular. Septothecal thickenings present or absent. Endothecal dissepiments vesicular in peripheral corallite areas and subtabulate in axial corallite areas. Epithecal sensu lato wall folded.
Placophyllia cf. dianthus (Goldfuss, 1826)
Figs. 8A–C
A Placophyllia cf. dianthus (Goldfuss, 1826), NMSG Coll. PK 02.10.26a; calicular view, polished surface; scale bar: 2.5 mm. B Placophyllia cf. dianthus (Goldfuss, 1826), NMSG Coll. PK 02.10.26a; calicular view, thin section; scale bar: 2.5 mm. C Placophyllia cf. dianthus (Goldfuss, 1826), NMSG Coll. PK 02.10.27a; calicular view, polished surface; scale bar: 2.5 mm. D Placophyllia cf. florosa Eliášová, 1976b, NMSG Coll. PK 02.10.28a; calicular view, polished surface; scale bar: 3 mm. E Placophyllia cf. florosa Eliášová, 1976b, NMSG Coll. PK 02.10.31a; calicular view, thin section; scale bar: 2.5 mm. F Dermosmilia sp., NMSG Coll. PK 02.10.32a; calicular view, thin section; scale bar: 3.5 mm. G Fungiastraea lamellosa (de Fromentel, 1857), NMSG Coll. PK 02.10.16b; upper surface of colony, calicular view; scale bar: 4 mm. H Fungiastraea lamellosa (de Fromentel, 1857), NMSG Coll. PK 02.10.28h; calicular view, thin section; scale bar: 2 mm. I Actinaraea tenuis Morycowa, 1971, NMSG Coll. PK 02.10.30e; calicular view of colony, thin section; scale bar: 2 mm. J Actinaraea tenuis Morycowa, 1971, NMSG Coll. PK 02.10.28e; calicular and longitudinal view of colony, polished surface; scale bar: 3.5 mm. K Latiastrea mucronata Sikharulidze, 1979, NMSG Coll. PK 02.10.22 g; calicular view of colony, polished surface; scale bar: 3 mm. L Latiastrea mucronata Sikharulidze, 1979, NMSG Coll. PK 02.10.22 g; close-up of Fig. M, scale bar: 1 mm. M Latiastrea mucronata Sikharulidze, 1979, NMSG Coll. PK 02.10.22 g; calicular view, thin section; scale bar: 2 mm
-
v*1826 Lithodendron dianthus: Goldfuss, p. 45, Pl. 3, Fig. 8.
-
v1876 Placophyllia? rugosa Beck.: Becker, p. 140, Pl. 38, Fig. 9a–b (older synonyms cited therein).
-
1985 Placophyllia dianthus (Goldfuss, 1826): Rosendahl, p. 49, Pl. 1, Fig. 10.
-
1989 Placophyllia cf. dianthus (Goldfuss): Beauvais, p. 295.
-
1990 Placophyllia rugosa Becker, 1876: Eliášová, p. 121, pl. 2, Fig. 1.
-
v1991 Placophyllia dianthus (Goldfuss, 1826): Lauxmann, p. 155, Text–Fig. 15.
-
v1997 Placophyllia rugosa Becker, 1876: Turnšek, p. 153, Figs. 153A–F.
-
2003 Placophyllia dianthus (Goldfuss, 1826): Kołodziej, p. 213, Fig. 27 [older synonyms cited therein].
-
2005 Placophyllia cf. dianthus (Goldfuss, 1826): Morycowa & Mišik, 2005, p. 420, Fig. 3.5.
-
2008 Placophyllia rugosa Becker, 1876: Roniewicz, p. 104, Figs. 6E–F.
-
v2018 Placophyllia dianthus (Goldfuss, 1826): Baron-Szabo, p. 48, Pl. 5, Fig. A–B, D–E [older synonyms cited therein].
Dimensions of skeletal elements. Diameter of corallites: 7–9 mm; number of septa: 32–36.
Description. Fragments of a phaceloid colony; corallites subcircular to subpolygonal in outline; costosepta developed in 3 to 4 size orders; lamellar columella generally attached to septa at either one or both ends of longer axis; septothecal thickenings present frequently but peripheral area of corallite often abraded.
Type locality of species. Upper Jurassic of Germany (Giengen).
Distribution. Upper Jurassic of Germany and Sumatra, Oxfordian of Slovakia, upper Oxfordian–lower Kimmeridgian of Poland, upper Oxfordian–Kimmeridgian of Georgia (in Caucasus), Russia, and Slovenia, lower Kimmeridgian of Portugal, Kimmeridgian of Croatia, Kimmeridgian–Berriasian of Bulgaria, upper Tithonian–lower Berriasian of the Czech Republic (Štramberk limestone) and Poland (Štramberk-type limestone), upper Berriasian (upper Őhrli Formation [= Oerfla Formation]) of western Austria and eastern Switzerland, lower Valanginian of northeastern Switzerland (Vitznau Marl, Wart; this paper), ?Valanginian of Poland (according to Kołodziej [2003, p. 213], material belonging to P. dianthus was collected from sediments of the Sub-Silesian Nappe, Outer Carpathians, which have a questionable Valanginian age).
Material. NMSG Coll. PK–02.10.22b; –02.10.23a (= 02.10.29a); –02.10.26a; –02.10.27a.
Remarks.
Because the Swiss material represents only small, fragmented colonies, the total dimensions of skeletal elements cannot be determined.
Placophyllia cf. florosa Eliášová, 1976b
Figs. 8D–E
-
v*1976b Placophyllia florosa n. sp: Eliášová, p. 339, Pl. 3, Figs. 1–2.
-
2018 Placophyllia cf. florosa Eliášová, 1976: Ricci, et al., 2018, p. 451–453, Pl. 7, Fig. 1a–1c.
Dimensions of skeletal elements. Diameter of corallites: 14–19 mm; number of septa: up to around 48.
Description. Fragments of a phaceloid colony; corallites subcircular to subpolygonal in outline; costosepta developed in 3 to 4 size orders; lamellar columella often thick, generally attached to septa at either one or both ends of longer axis; septothecal thickenings present frequently but peripheral area of corallite often abraded.
Type locality of species. Tithonian–lower Berriasian of the Czech Republic (Štramberk limestone).
Distribution. Kimmeridgian–Tithonian of Italy, Tithonian–lower Berriasian of the Czech Republic (Štramberk limestone), lower Valanginian of northeastern Switzerland (Vitznau Marl, Wart; this paper).
Material. NMSG Coll. PK–02.10.22c; –02.10.28a; –02.10.28b (= 02.10.30b); –02.10.31a.
Remarks.
Because the Swiss material represents only small, fragmented colonies, the total dimensions of skeletal elements cannot be determined.
Family Dermosmiliidae Koby, 1887
Genus Dermosmilia Koby, 1884
Type species. Dermosmilia crassa Dacqué, 1933, Upper Jurassic (Rauracian) of Switzerland (subsequent designation Dacqué, 1933).
Diagnosis. Colonial, dendroid, phaceloid, fasciculate, subflabellate. Corallites nearly circular to subflabellate in outline. Budding intracalicular, di- to polystomodaeal, complete. Corallites united only basally. Costosepta generally compact to subcompact, sometimes irregularly perforated, straight or irregularly wavy, laterally granulated. Anastomosis present or absent. When present, septa may be arranged during various stages of ontogeny in a pattern resembling the kinds seen in micrabaciid or dendrophylliid genera (similar to Pourtalès plan). Columella spongy-papillose or formed by fusion of trabecular prolongations of axial ends of septa. Synapticulae present. Intertrabecular distance ranging between 80 and 300 µm. Endothecal dissepiments thin, vesicular to subtabulate. Wall parathecal to parasynapticulothecal, often secondarily thickened.
Remarks.
The above given diagnosis for Dermosmilia is based on both the study of material from Caquerelle and Sante-Ursanne strata from various localities of the Koby collections housed at the museums in Basel and Bern, and the information provided by Koby (1884, p. 194–195, Pl. 50, Figs. 1–6).
Dermosmilia sp.
Fig. 8F
Dimensions. Diameter of corallite: 12 × 17 mm; septa/corallite: around 80.
Description. Fragment of a branching colony; corallite elongate in outline; septa thin, nearly equal in thickness, rather straight, developed in unclear size orders.
Material. NMSG Coll. PK–02.10.32a.
Remarks.
Because only one corallite is available, the branching angle cannot be determined. Based on the dimensions seen in the Swiss material, it could belong to species such as D. laxata, D. etalloni, or D. arborescens.
Family Haplaraeidae Vaughan & Wells, 1943
Genus Actinaraea d’Orbigny, 1849
Type species. Agaricia granulata Münster, in Goldfuss, 1829, Upper Jurassic of Germany (Nattheim).
Diagnosis. Colonial, massive, folios, thamnasterioid, including ploco- to cerio-thamnasterioid. Budding intracalicular (-marginal). Corallites embedded in a coenosteum that is generally porous to reticulate. Costosepta few in number with irregular perforations, septal flanks granular. No paliform structures. Columella generally feebly developed, parietal, lamellar, substyliform. Synapticulae present. Intertrabecular distance ranging between 80 and 180 µm. Endothecal dissepiments thin, tabulate. Wall absent or incomplete synapticulothecal.
Subgenus. Camptodocis Dietrich, 1926 (Type species. C. brancai Dietrich, 1926, Barremian–lower Aptian of Tanzania): Having the characteristics of Actinaraea but calices are not independent from perithecal colony tissue (similar as in Actinacis), corallites therefore with variably ploco- to cerio-thamnasterioid integration types.
Actinaraea tenuis Morycowa, 1971
Figs. 8I–J
-
(v)*1971 Actinaraea tenuis n. sp.: Morycowa, p. 128–130, Pl. 35, Fig. 1a–d, Pl. 36, 1a–c, Text-Fig. 37.
-
1980 Actinaraea tenuis Morycowa, 1971: Kuzmicheva, p. 106–107, Pl. 39, Fig. 4a–b.
-
non1984 Actinaraea sp. aff. A. tenuis Morycowa, 1971: Scott, p. 344, Pl. 2, Figs. 12–13.
-
1992 Actinaraea cf. A. tenuis Morycowa, 1971: Turnšek, 1992, p. 164, Fig. 2 .
-
v1996 Actinaraea tenuis Morycowa, 1971: Wilmsen, 1996, p. 361, Pl. 4, Fig. 3.
-
1996 Actinaraea tenuis Morycowa, 1971: Császár & Turnšek, p. 430, Fig. 7(6).
-
v1997 Actinaraea tenuis Morycowa, 1971: Baron-Szabo, p. 79, Pl. 12, Fig. 1–2 [older synonyms cited therein].
-
2003 Actinaraea tenuis Morycowa, 1971: Turnšek, et al., p. 179, Figs. 12A–B.
-
v2014 Actinaraea tenuis Morycowa, 1971: Baron-Szabo, p. 53, Pl. 58, Fig. 3 Pl. 59, Fig. 1–2.
-
v2021b Actinaraea tenuis Morycowa, 1971: Baron-Szabo, p. 54, Pl. 8, Fig. A.
Dimensions. Corallite diameter: 1–1.7 mm; distance of corallite centers: 2.3–4.5 mm; septa/corallite: up to around 24; septa/mm: 6–8/2; synapticulae (longitudinal view)/mm: 3–4/1.
Description. Submassive to foliose, thamnasterioid colony; corallites subdistinct to indistinct, regularly disposed over the colony; septa confluent to subconfluent; 6–8 septa reach corallite center; columella substyliform or made of a small number of thin, twisted segments.
Type locality of species. Lower Aptian of Romania (Valea Izvorul Alb).
Distribution. Lower Valanginian of northeastern Switzerland (Vitznau Marl, Wart; this paper), Valanginian of Hungary, Hauterivian of Georgia (in Caucasus), Barremian–lower Aptian of Serbia, Barremian–Aptian of Slovenia, upper Barremian–lower Aptian of western Austria (Schrattenkalk Formation, Vorarlberg), lower Aptian of Romania and southern Germany (Upper Schrattenkalk, Bavaria), middle Albian of the USA (New Mexico), lower Cenomanian of Spain, lower Coniacian of Austria (Gosau Group at Brandenberg).
Material. NMSG Coll. PK–02.10.14b; –?02.10.16a; –02.10.23e (= 02.10.29e); –02.10.26c; –02.10.26c-1; –02.10.28e; –02.10.30e; –02.10.37a.
Remarks.
Information regarding material of Scott (1984) see Appendix Table 5.
Family Latomeandridae Alloiteau, 1952
Genus Fungiastraea Alloiteau, 1952
Type species. Fungiastraea laganum Alloiteau, 1952, Upper Turonian of France (Uchaux, Vaucluse).
Diagnosis. Colonial, massive, thamnasterioid to submeandroid. Budding intracalicular, occasionally extracalicular. Corallite centers distinct. Septa compact to subcompact, confluent, moderately granulated and pennulated laterally. Sub- to non-confluent septa sparse, occurring in areas of extracalicular budding. Columella spongy, papillose, or variably shaped when columellar trabeculae fuse. Paliform structures absent. Synapticulae present. Intertrabecular distance ranging between 90 to around 350 µm. Endothecal dissepiments thin, vesicular to subtabulate. No wall between corallites but parasynapticulothecal developments circumscribing some parts of corallites sometimes present.
Fungiastraea lamellosa (de Fromentel, 1857 )
Figs. 8G–H
-
v*1857 Thamnastraea lamellosa: de Fromentel, p. 61.
-
v1887 Centastraea lamellosa: de Fromentel, 1887, p. 617, Pl. 187, Fig. 1–1c .
-
1914Centastraea lamellosa: de Fromentel: Felix, 1914, p. 55.
-
non1998 Fungiastraea lamellosa (de Fromentel, 1857): Löser, p. 180.
-
non2001 Dimorphastrea cf. lamellosa (de Fromentel, 1857): Löser, p. 46.
Dimensions. Diameter of corallites (monocentric): 2–3 mm, in areas of intense budding around 1.5 mm; distance of corallite centers: 2–4 mm; septa/corallite: 18–24 + s, in corallites in areas of intense budding around 12; septa/mm: 5–8/2.
Description. Thamnasterioid-submeandroid colony; corallites irregularly disposed or arranged in short-meandroid series; septa subequal in thickness; up to 6 septa reach corallite center; columella made of a small number of papillae or twisted segments.
Type locality of species. Lower Hauterivian of France (Yonne).
Distribution. Lower Valanginian of northeastern Switzerland (Vitznau Marl, Wart; this paper), lower Hauterivian of France (Yonne).
Material. NMSG Coll. PK–02.10.16b; –02.10.28h.
Remarks.
In having larger dimensions of skeletal elements (corallite diameter: 2.5–4 mm; septa/corallite: 25–30; septa/mm: 8/2), the material from the lower Cenomanian of Germany provisionally assigned to lamellosa in Löser (1998) differs from de Fromentel’s species. In forming a dimorphastreid corallum and having smaller dimensions of skeletal elements (septa/corallite: 16–20 (24); septa/mm: 5–6/2), the material from the lower Hauterivian of France provisionally assigned to the species lamellosa in Löser (2001) differs from de Fromentel’s species. The Swiss material corresponds well to the syntype of the species F. lamellosa (MNHN.F.M03558).
Genus Latiastrea Beauvais, 1964
Type species. Latiastrea foulassensis Beauvais, 1964, Upper Jurassic (Kimmeridgian) of France (Valfin-les-Saint-Claude).
Diagnosis. Colonial, massive, cerioid to meandroid. Budding intracalicular. Corallites prismatic, elongate, monocentric, or temporarily dicentric (to ?polycentric) during budding processes, or arranged in meandroid series. Costosepta non-confluent to subconfluent, with rare perforations on axial ends of septa. Anastomosis present. Rudimentary young septa alternate with old ones. Septal flanks are ornamented with large, spiniform granulae. Pennulae present. Distal margins covered with small, regularly developed rounded denticles. Synapticulae present. Columella parietal-spongy, sometimes forming elongate segments. Intertrabecular distance ranging between 100 to around 200 µm. Endothecal dissepiments thin, vesicular. Wall synapticulothecal and septothecal.
Latiastrea mucronata Sikharulidze, 1979
Figs. 8K–M.
-
(v)*1979 Latiastraea mucronata Sikh., sp. nov.: Sikharulidze, p. 37, Pl. 3, Fig. 4–4a, Pl. 23, Pl. 24, Fig. 1a–b.
-
1996 Latiastraea mucronata Sikharulidze, 1979: Császár & Turnšek, p. 434, Fig. 26.
-
v1999 Latiastraea mucronata Sikharulidze, 1979: Baron-Szabo & González-León, p. 490, Fig. 6e.
-
(v)2002 Latiastrea mucronata Sikharulidze, 1979: Morycowa & Marcopoulou-Diacantoni, p. 53, Figs. 34F, G.
-
v2003 Latiastraea mucronata Sikharulidze, 1979: Baron-Szabo & González-León, p. 220, Fig. 9D, F.
-
v2018 Latiastrea mucronata Sikharulidze, 1979: Baron-Szabo, p. 66, Pl. 9, Fig. A.
Dimensions of skeletal elements. Diameter of corallite (monocentric): 2–4 mm; distance of corallite centers: 2–4.5 mm; septa/corallite (monocentric): up to around 30; septa/mm: 7–8/2.
Description. Massive, cerioid colony; septa are subequal in thickness, irregularly alternate in length; columella often made of elongate segments.
Type locality of species. Albian of Georgia (in Caucasus).
Distribution. Upper Berriasian (upper Őhrli Formation [= Oerfla Formation]) of western Austria, lower Valanginian of northeastern Switzerland (Vitznau Marl, Wart; this paper), Valanginian of Hungary, upper Aptian–lower Albian of Mexico, Albian of Georgia (in Caucasus) and Greece.
Material. NMSG Coll. PK–02.10.22e (= 02.10.30a); –02.10.22g.
Genus Thamnoseris de Fromentel, 1861
Type species. Thamnoseris incrustans de Fromentel, 1861, Middle Jurassic of France (Chaumont, Saint Claude, French Jura).
Diagnosis. Colonial, massive, hemispherical, cerio-thamnasterioid, corallites arranged in short-meandroid series present or absent. Budding extracalicular-marginal. Costosepta confluent, irregularly perforated, granulate and probably pennulate laterally. Anastomosis frequently present. Columella parietal-papillose. Synapticulae numerous. Endothecal dissepiments vesicular, thin. Intertrabecular distance ranging between 120 and ca. 240 µm. Wall synapticulothecal, incomplete.
Thamnoseris cf. carpathica Morycowa, 1971
Figs. 9A–B
A Thamnoseris cf. carpathica Morycowa, 1971, NMSG Coll. PK 02.10.37b; calicular view of colony, polished surface; scale bar: 2 mm. B Thamnoseris cf. carpathica Morycowa, 1971, NMSG Coll. PK 02.10.37b; oblique longitudinal view of colony, polished surface; scale bar: 1 mm. C Adelocoenia parvistella Alloiteau, 1961, NMSG Coll. PK 02.10.29d (= 23d); calicular view of colony, thin section; scale bar: 2 mm. D Adelocoenia parvistella Alloiteau, 1961, NMSG Coll. PK 02.10.29d (= 23d); close-up of Fig. C; scale bar: 1 mm. E Adelocoenia parvistella Alloiteau, 1961, NMSG Coll. PK 02.10.23d (= 29d); upper surface of colony, calicular view, partially polished; scale bar: 2 mm. F Pleurophyllia ? tobleri (Koby, 1896), NMSG Coll. PK 02.10.15; calicular view of corallum, polished surface; scale bar: 1 mm. G Pleurophyllia ? tobleri (Koby, 1896), NMSG Coll. PK 02.10.20d; calicular view of corallum, upper surface; scale bar: 1 mm. H Cyathophora claudiensis Étallon, 1859, NMSG Coll. PK 02.10.22a; calicular view of colony, thin section; scale bar: 4 mm. I Cyathophora claudiensis Étallon, 1859, NMSG Coll. PK 02.10.22a; upper surface of colony, calicular view; scale bar: 10 mm. J Cyathophora claudiensis Étallon, 1859, NMSG Coll. PK 02.10.29b (= 23b); calicular view of colony, thin section; scale bar: 3 mm. K Stylophyllid indet., NMSG Coll. PK 02.10.14c (=13) longitudinal view of corallum, oblique, polished surface; green arrows indicate septa; orange arrow indicates corallite wall; scale bar: 4 mm. L Stylophyllopsis silingensis (Liao, 1982), NMSG Coll. PK 02.10.35l; calicular view of colony, thin section; scale bar: 2 mm
-
v*1971? Thamnoseris carpathica n. sp.: Morycowa, p. 106–108, Pl. 28, Fig. 1a–e.
-
nonv1981 Thamnoseris carpathica Morycowa, 1971: Turnšek, in Turnšek & Mihajlović, p. 29, Pl. 31, Fig. 4–5.
-
1988 Thamnoseris carpathica Morycowa, 1971: Kuzmicheva & Aliev, 1988 p. 169, Pl. 5, Fig. 4a–b.
-
2006 Thamnoseris carpathica Morycowa, 1971: Morycowa & Decrouez, p. 810–812, Pl. 9, Figs. 5–7. [older synonyms cited therein]
-
2021b Thamnoseris carpathica Morycowa, 1971: Baron-Szabo, Appendix Tables 2–5, and 7–12.
Dimensions. Diameter of corallites: 2–4.5 mm, in areas of intense budding around 1.5 mm; distance of corallite centers: 2.5–5 mm, in areas of intense budding the distance is around 1.5 mm; septa/corallite: 20 to around 32, in corallites in areas of intense budding 12; septa/mm: 6–7/2.
Description. Fragment of a massive, cerio-thamnasterioid colony; corallites isolated or arranged in short-meandroid series; septa subequal in thickness; up to around 12 septa reach corallite center.
Type locality of species. Lower Aptian of Romania (Valea Izvorul Alb).
Distribution. Lower Valanginian of northeastern Switzerland (Vitznau Marl, Wart; this paper), Barremian of Azerbaijan, lower Aptian of Romania (Valea Izvorul Alb) and Switzerland (Upper Schrattenkalk of Hergiswil).
Material. NMSG Coll. PK–02.10.37b.
Remarks.
The material described as Thamnoseris carpathica from the Barremian–lower Aptian of eastern Serbia (Turnšek & Mihajlović, 1981) has just recently been transferred to the genus Thalamocaeniopsis, grouping it with the species Th. stricta (Milne Edwards & Haime) (see Baron-Szabo, 2021b). Because the Swiss material represents only a fragment of a colony, the total dimensions of the skeletal elements cannot be determined. The skeletal features observed correspond to the species Th. carpathica.
Family Stylinidae d’Orbigny, 1851
Genus Adelocoenia d’Orbigny, 1849
Type species. Astrea castellum Michelin, 1844, Upper Jurassic of France (neotype designation Lathuilière, et al., 2020).
Diagnosis. Colonial, massive to subhemispherical, small knobby, plocoid corallum. Septa compact, free, bicuneiform, costate, non-confluent, often straight, unequal in length, arranged mainly radially; bilateral arrangement might occur as a result of elongation of both calices and calicular fossae. Septal ornamentation very weak or smooth when covered by thickening deposits. Secondary trabecular axes irregularly emerge from the mid-septal plan toward the septal faces. Pali and synapticulae absent. Endotheca made of tabulae or tabuloid dissepiments, rarely vesicular. Peritheca made of vesicular dissepiments. Columella absent but a clear central subcircular fossa present (in neotype of type species, in one out of 32 corallites, however, structures are present which may or may not correspond to a columella). Intertrabecular distance up to around 100 µm. Wall parathecal, developed in continuity with thickening deposits of septa.
Adelocoenia parvistella Alloiteau, 1961
Figs. 9C–E
-
v*1961 Adelocoenia parvistella: Alloiteau, p. 289, pl. 9 fig. 5; pl. 10 fig. 9.
-
v1972 Pseudocoenia slovenica n. sp.: Turnšek, 1972: p. 20 and 83, Pl. 4, Fig. 1–2, Pl. 5, Figs. 1–4.
-
1976 Pseudocoenia slovenica Turnšek, 1973: Roniewicz, p. 48, pl. 5 fig. 5.
-
1981 Pseudocoenia slovenica Turnšek, 1972: Eliášová, 1981 p. 125, pl. 8 figs. 3–4.
-
1985 Pseudocoenia slovenica Turnšek: Rosendahl, p. 34, pl. 3 fig. 2.
-
1990 Pseudocoenia slovenica Turnšek, 1972 Errenst, p. 168, pl. 3 fig. 1.
-
v1997 Pseudocoenia slovenica Turnšek, 1972: Turnšek, p. 171, Figs. A–F.
-
2001 Pseudocoenia cf. slovenica Turnšek, 1972: Laternser, 2001 p. 162.
-
2002 Pseudocoenia slovenica Turnšek, 1972 Kashiwagi, et al., 2002 p. 10, fig. 5.2.
-
pars?2003 Pseudocoenia slovenica Turnšek, 1972: Pandey & Fürsich, p. 27, pl. 5 fig. 5; pl. 6 fig. 1–6.
-
non2003 Pseudocoenia cf. slovenica Turnšek, 1972: Pandey & Fürsich, p. 27, pl. 4 fig. 4.
-
2015 Pseudocoenia slovenica Turnšek: Kołodziej, 2015, p. 182.
-
v2020 Adelocoenia parvistella Alloiteau, 1961: Lathuilière, et al., p. 381–382, Figs. 15–16 [older synonyms cited therein]
Dimensions. Diameter of corallites: 1–1.8 mm; in areas of intense budding the diameter is around 0.8 mm; distance of corallite centers: 1.2–2 mm, in areas of intense budding the distance is around 0.8 mm; septa/corallite: 12 (6s1 + 6s2); costae/corallite: 12 and higher.
Description. Small massive to knobby colony; corallites circular to subcircular in outline, regularly disposed over the colony; costosepta developed in 2 complete cycles in 6 systems, regularly alternating in length and thickness; number of costae is equal to or slightly larger than number of septa; columella absent but in a small number of corallites trabecular extensions of axial ends of septa reach corallite center where they might fuse, forming a pseudo-columella.
Type locality of species. Upper Jurassic (Tithonian) of Spain (La Querola).
Distribution. Upper Jurassic of Armenia and Japan, Oxfordian of Germany and France, upper Oxfordian of Romania, Oxfordian–Kimmeridgian of Slovenia, lower Kimmeridgian of Romania, Kimmeridgian–Tithonian of Spain, Tithonian of Portugal, Tithonian–lower Berriasian of the Czech Republic and Poland, lower Valanginian of northeastern Switzerland (Vitznau Marl, Wart; new material this paper).
Material. NMSG Coll. PK–02.10.20f; –02.10.23d (= 02.10.29d); –02.10.27b; –02.10.28c and 28d; –02.10.29f; –02.10.30d; –02.10.32b.
Remarks.
In Adelocoenia parvistella, auriculae are present which clearly distinguishes it from genera such as Cyathophora (including its junior synonym Cryptocoenia). This represents an important fact, since Alloiteau (1958) also erected a species Cryptocoenia parvistella, using material from the Cretaceous of Madagascar (MNHN.F.M05039; and thin sections). Because the latter is here considered to belong to Cyathophora, the species is not a junior homonym.
Some of the specimens from the Jurassic of Iran assigned to this species A. slovenica by Pandey and Fürsich (2003, p. 27), are considered as Solenocoenia (such as material shown on their pl. 6, Fig. 5). In addition, it should be noted that these authors place within A. slovenica material having dimensions that significantly differ from A. slovenica (e.g., specimen SNSB-BSPG 1999 VIII 874: corallite diameter: 1.5–2.3 mm; and specimen SNSB-BSPG 1999 VIII 1085: corallite diameter: 1.5–2.6 mm). The consequence of such a wide grouping would be a much wider stratigraphic range for this species.
Family Amphiastreidae Ogilvie, 1897
Genus Pleurophyllia De Fromentel, 1856
Type species. Pleurophyllia trichotoma De Fromentel, 1856, Upper Jurassic of France (Mantoche).
Diagnosis. Colonial, phaceloid. Budding intracalicinal. Septa compact, smooth laterally, arranged bilaterally. One major septum extends to the axial region of the corallite. Septal cycles indistinct. Costae and columella absent. Intertrabecular distance ranging between 90 and ca. 160 µm. Endothecal dissepiments thin. Multilamellate epithecal sensu lato wall possibly present.
Pleurophyllia ? tobleri (Koby, 1896 )
Figs. 9F–G
-
parsv*1896 Cladophyllia Tobleri, Koby, 1896: Koby, p. 42, Pl. 7, Fig. 5 (non Fig. 4–4a)
-
2008 Pleurophyllia aff. cara Eliášová, 1975: Roniewicz, p. 97, Fig. 3B.
-
v2014 Pleurophyllia tobleri (Koby, 1896): Baron-Szabo, p. 82, Text–Fig. 21.
-
v2018 Pleurophyllia tobleri (Koby, 1896): Baron-Szabo, p. 81, Pl. 12, Figs. C–D [older synonyms cited therein].
Dimensions. Corallite diameters: 4–6 mm; septa/corallite (monocentric): around 16.
Description. Fragments of a branching colony; corallites very elongate in outline; septa arranged bilaterally, irregularly alternate in length and thickness.
Type locality of species. Upper Berriasian (upper Őhrli Formation) of Switzerland (Canton Uri: Schöner Culm).
Distribution. Upper Berriasian (upper Őhrli Formation; Canton Uri: Schöner Culm)–lower Valanginian of northeastern Switzerland (Vitznau Marl, Wart; this paper), Valanginian of Bulgaria.
Material. NMSG Coll. PK–02.10.15; –02.10.20d.
Remarks.
Because only colony fragments are available, the total range of dimensions of skeletal elements cannot be determined. The skeletal elements that are present correspond well to the species P. tobleri.
Family Cyathophoridae Vaughan & Wells, 1943
Genus Cyathophora Michelin, 1843
Type species. Cyathophora richardi Michelin, 1843, Upper Jurassic of France (lectotype designation Zaman & Lathuilière, 2014).
Diagnosis. Colonial, massive, columnar, hemispherical, knobby, plocoid, cerio-plocoid to cerioid in areas of closely spaced corallites. Budding extracalicular. Corallites circular to subpolygonal in outline, separated by a narrow costate peritheca. Costosepta compact, generally non-confluent to subconfluent, occasionally confluent, radially arranged. Sizes of septa range from very short (less of a quarter of the lumen size) to half the lumen size, in which case septa reach the axial region where they sometimes fuse. Axial ends of S1 are vertically discontinuous. No columella. No synapticulae. No pali. Endothecal and exothecal dissepiments tabulate, well-developed. Wall parathecal and septothecal.
Cyathophora claudiensis Étallon, 1859
Figs. 9H–J
-
*1859 Cyathophora claudiensis: Étallon, p. 479.
-
1897 Cyathophora claudiensis Ét.: Ogilvie, p. 176, Pl. 16, Figs. 11–12.
-
v1954 Cyathophora claudiensis Étallon, 1859: Geyer, 1954 p. 137, Pl. 9, Fig. 12.
-
1970 Amphiphora serannensis nov. sp.: Alloiteau & Bernier, 1970 p. 926–927, Pl. 28, Figs. 1–3.
-
1976 Cyathophora claudiensis Étallon, 1859: Roniewicz, p. 44–45, Pl. 4, Fig. 1–b [older synonyms cited therein].
-
1982 Cyathophora claudiensis Étallon, 1859: Bendukidze, 1982 p. 7, Pl. 1, Fig. 5.
-
nonv1991 Cyathophora claudiensis Étallon, 1859: Lauxmann, p. 114–115.
-
(v)2008 Cyathophora sp. 1: Roniewicz, p. 128, Figs. 16A–D.
-
2015 Cyathophora claudiensis: Kołodziej, 2015 p. 183.
Dimensions. Great diameter of corallites: 4.5–7 mm, in areas of intense budding around 3.5 mm; distance of corallite centers: 6–9 mm, in areas of intense budding the distance is around 4 mm; septa/corallite: 6 + 6 + 12.
Description. Massive to subhemispherical, plocoid to cerio-plocoid colony; corallites are circular to elongate in outline; costosepta are very short and spine-like, developed in two to three complete cycles in 6 systems.
Type locality of species. Upper Jurassic of France (Valfin).
Distribution. Upper Jurassic of France, upper Oxfordian of Poland and Russia, lower Kimmeridgian of Romania, lower Tithonian of Russia, Tithonian–Berriasian of Germany, Tithonian–lower Berriasian of the Czech Republic and Poland (Štramberk-type limestones at Polish Outer Carpathians), Tithonian and Valanginian of Bulgaria, lower Valanginian of northeastern Switzerland (Vitznau Marl, Wart; this paper),
Material. NMSG Coll. PK–02.10.22a; –02.10.23b (= 02.10.29b); –02.10.28g; –02.10.30c; –02.10.34.
Remarks.
The dimensions of skeletal elements in the material reported from the Tithonian and Valanginian of Bulgaria (Roniewicz, 2008, p. 128, Figs. 16A–D) seem to be slightly larger than given in the original description (= corallite diameter: 5–6 mm; septa: 6 + 6 + nS3; but material shown on Figs. 16A–D seems to have corallite diameters of up to 7 mm and 24 septa), thus closely corresponding to C. claudiensis.
In having septa developed in more than 3 complete cycles in 6 systems, the material described from the Upper Jurassic of southern Germany as C. claudiensis in Lauxmann (1991) differs from the species claudiensis and more closely corresponds to the species C. bourgueti.
Family Stylophyllidae Frech, 1890
Genus Stylophyllopsis Frech, 1890
Type species. Stylophyllopsis polyactis Frech, 1890, Upper Triassic of Austria (Rhaetian, Zlambach Beds) (lectotype designation Roniewicz, 1989).
Diagnosis. Solitary and phaceloid. Budding intracalicular. Septal spines long, connected by stereome, resulting in subcompact septal blades that have large pores. Additional stereome layers might fuse the septal spines and cover endothecal dissepiments. In the septal spines axial rods or lamellae are present. Axial ends of septa dissociate, forming septal spines. Distal edge of septa coarsely denticulate. Columella formed by isolated septal spines. Endothecal dissepiments vesicular to subtabulate; large and vesicular in peripheral area of corallite. Peripheral, large-vesicular dissepimentarium irregularly present. Microstructure made of bundles of fiber. Epitheca sensu lato present.
Stylophyllopsis silingensis (Liao, 1982 )
A Heterocoenia inflexa (Eichwald, 1865–69), NMSG Coll. PK 02.10.31d; calicular view of colony, polished surface; scale bar: 1.25 mm. B Heterocoenia inflexa (Eichwald, 1865–69), NMSG Coll. PK 02.10.31d; upper surface of colony, calicular and oblique views; scale bar: 8 mm. C Aplosmilia semisulcata (Michelin, 1843), NMSG Coll. PK 02.10.29h; calicular view of branch, polished surface; scale bar: 3.5 mm. D Aplosmilia semisulcata (Michelin, 1843), NMSG Coll. PK 02.10.29h; calicular view of branch, thin section; scale bar: 3 mm. E Aplosmilia semisulcata (Michelin, 1843), NMSG Coll. PK 02.10.29h; calicular view of corallites in advanced budding stage, polished surface; scale bar: 3 mm. F Stylophyllopsis silingensis (Liao, 1982), NMSG Coll. PK 02.10.25b; calicular view of branch, slightly oblique, polished surface; scale bar: 2 mm. G Stylophyllopsis silingensis (Liao, 1982), NMSG Coll. PK 02.10.29c (= 23c); calicular view of branch, slightly oblique, polished surface; scale bar: 2 mm. H Stylophyllopsis silingensis (Liao, 1982), NMSG Coll. PK 02.10.35l; close-up of Pl. 3, Fig. L; scale bar: 6 mm. I Axosmilia villersensis (Koby, 1898), NMSG Coll. PK 02.10.10; calicular view of corallum, thin section; younger cycle septa are preserved in only some parts of the corallum; scale bar: 3 mm. J Stylophyllopsis silingensis (Liao, 1982), NMSG Coll. PK 02.10.35h; calicular view of juvenile corallite, thin section; scale bar: 1 mm
-
*1982 Donacosmilia silingensis Liao (sp. nov.): Liao, p. 166, Pl. 14, Figs. 1–2.
-
1994 Donacosmilia silingensis Liao: Liao & Xia, p. 78, Pl. 8, Figs. 5–6.
Dimensions. Great diameter of corallites: 6–11 mm; small diameter of corallites: 5–7 mm; septa/corallite: 32 to around 46.
Description. Phaceloid colony; corallites subcircular to elongate in outline; septa generally straight, arranged bilaterally in 4 to 5 size orders, irregularly alternating in length and thickness; up to around 20 septa reach corallite center; columella weakly developed.
Type locality of species. Hauterivian of Tibet (Xainza County; Toiba Formation).
Distribution. Lower Valanginian of northeastern Switzerland (Vitznau Marl, Wart; this paper), Hauterivian of Tibet.
Material. NMSG Coll. PK–02.10.23c (= 02.10.29c); –02.10.25b; –02.10.35c (= 02.10.35f and 35f-II); –02.10.35d (= 02.10.35g); –02.10.35e (= 02.10.35h); –02.10.35l.
Remarks.
Study of the lectotype of the type species of Stylophyllopsis Frech (S. polyactis [SNSB-BSPG-AS-XII-53]) in 2020 by one of the authors (RBS) revealed that, in addition to previously reported skeletal features, it also shows a peripheral, large-vesicular dissepimentarium in some places.
In having (1) septa made of spines that are connected or dissociated; (2) large and vesicular dissepiments in the peripheral area of the corallite; (3) an epithecal sensu lato wall; (4) axial ends of septa that dissociate, forming septal spines; (5) a columella formed by isolated septal spines; and (6) lamellae that occur in some of the septal spines, the material described as Donacosmilia silingensis from the Hauterivian of Tibet closely corresponds to the genus Stylophyllopsis. The Swiss specimens show very close resemblance to the Tibetan type material as presented in Liao (1982) and Liao and Xia (1994).
Stylophyllid indet.
Fig. 9K
Dimensions. ?Great diameter of corallum: 10–22 mm; septa/ mm: 6–7/2.
Description. Fragments of ?solitary coralla showing stylophyllid structures; corallites subcircular to elongate in outline; septa developed in probably 5 size orders which irregularly alternate in thickness; the material might belong to solitary genera such as Macgeopsis.
Material. NMSG Coll. PK–02.10.13 (= 02.10.14a); –02.10.17; –02.10.18a (= 02.10.19).
Remarks.
Because only incomplete specimens are available, neither the full range of dimensions of skeletal elements nor whether they belong to solitary or colonial forms can be determined. However, based on characteristics that can be identified, the material shows correspondence to solitary taxa such as Macgeopsis.
Family Heterocoeniidae Oppenheim, 1930
Genus Heterocoenia Milne Edwards & Haime, 1848b
Type species. Lithodendron exiguum Michelin, 1847, Santonian of France.
Diagnosis. Colonial massive, hemispherical, foliose, encrusting, ramose, plocoid, cerio-plocoid, (sub-) fasciculate; subphaceloid to reptoid when developments of coenosteum reduced. Budding extracalicular, extracalicular-marginal, and by septal division often dividing the corallite in three new ones (“trinity-arrangement” sensu Baron-Szabo, 2014). Corallites circular to elongate or irregularly polygonal in outline. They are directly united by their walls, or separated by extensive vesicular to dense coenosteum, or loosely connected by fragments of exotheca in form of traverses. Septa compact, arranged in various symmetries (e.g., trimerally, hexamerally, bilaterally, indistinct). One main septum, with remaining septa sometimes reduced to rudimentary spines. Costate zone present, weakly developed, or absent. Colony surface granulated or smooth. Columella, synapticulae, and paliform structures absent. Intertrabecular distance ranging between 60 and 120 µm in septa. Endothecal dissepiments thin, mainly tabulate to subtabulate, vesicular (often in peripheral and thecal areas). Exothecal dissepiments large vesicular. Wall often thick, possibly septothecal and paraseptothecal. Corallite wall tends to become more flaky, “bubbly” (cf. sclerenchymal deposits sensu Kołodziej, 1995, p. 4), and disintegrated during the process of budding.
Heterocoenia inflexa (Eichwald, 1865–69)
Figs. 10A–B
-
*1865–69 Stereopsammia inflexa m.: Eichwald, vol. 2, p. 164, Pl. 11, Fig. 2a–b.
-
1888 Latusastraea provincialis, d’Orb. sp.: Solomko, Geyer 1954 p. 76–77.
-
1907 Latusastraea ? inflexa Eichw.: Karakash, p. 262.
-
2002 Latusastraea exigua Fromentel, 1862: Kuzmicheva, p. 126, Pl. 8, Fig. 3.
-
v2018 Heterocoenia cf. inflexa (Eichwald, 1865–69): Baron-Szabo, p. 84, Pl. 12, Fig. G.
-
v2021b Heterocoenia inflexa (Eichwald, 1865–69): Baron-Szabo, p. 77, Pl. 13, Fig. F.
Dimensions of skeletal elements. Great diameter of corallites: 1–1.8 mm, up to around 3 mm when wall exceptionally thick (around 0.5 mm or larger); great diameter of corallites (lumen): 0.8–1.8 mm; septa/corallite: 1–12; distance of corallite centers: 1.2–3 mm, in areas of intense budding around 1 mm.
Description. Small submassive to subramose colony; corallites plocoid to cerio-plocoid, cerioid when crowded, oval to irregularly shaped in outline, regularly disposed over the colony; septa arranged bilaterally; in some corallites, septal arrangement in 3 systems present; one major septum present; remaining septa highly irregularly developed including very thin and half the length of major septum, or very short, spine-like; axial end of major septum rhopaloid or cuneiform; costae short and thin, up to twice the number of septa.
Type locality of species. Valanginian–lower Hauterivian of Ukraine.
Distribution. Upper Berriasian (upper Őhrli Formation [= Oerfla Formation]) of western Austria, lower Valanginian of northeastern Switzerland (Vitznau Marl, Wart; this paper), Valanginian–lower Hauterivian of Ukraine, upper Barremian–lower Aptian of western Austria (Schrattenkalk Formation, Vorarlberg), lower Aptian of southern Germany (Upper Schrattenkalk, Bavaria).
Material. NMSG Coll. PK–2.10.31d (= 2.10.32b).
Family Aplosmiliidae de Fromentel, 1861
Genus Aplosmilia d'Orbigny, 1849
Type species. Lobophyllia semisulcata Michelin, 1843, Jurassic of France (Verdun).
Diagnosis. Colonial, phaceloid. Corallites cylindrical to elliptical in outline. Budding intracalicular and? extracalicular. Septa compact, radially or bilaterally arranged, smooth distally. Lateral flanks of septa covered by sharp granulae. Flabelliform auriculae present. Lonsdaleoid and apophysal septa present or absent. Columella lamellar. Wall septoparathecal and parathecal. Pali and synapticulae absent. Intertrabecular distance ranging between 90 to around 250 µm. Endothecal dissepiments vesicular, mainly occurring in the vicinity of the wall, sometimes forming a stereozone. Tectura present or absent.
Aplosmilia semisulcata (Michelin, 1843 )
Figs. 10C–E
-
v*1843 Lobophyllia semisulcata. N.: Michelin, p. 89, Pl. 17, Fig. 8.
-
v1850 Aplosmilia semisulcata (Michelin, 1843): d’Orbigny, vol. 2, p. 37.
-
v1880 Aplosmilia semisulcata, Michelin, sp.: Koby, p. 50–51, Pl. 8, Figs. 3–4a, Pl. 14, Figs. 1–2a.
-
v1976 Aplosmilia semisulcata (Michelin, 1843): Roniewicz, p. 82–83, Pl. 20, Figs. 3a–d.
-
1985 Aplosmilia semisulcata (Michelin, 1843): Geyer & Rosendahl, p. 167, Pl. 2, Fig. 7.
-
1991 Aplosmilia semisulcata (Michelin, 1843): Errenst, p. 3–4, Pl. 13, Fig. 2a–b. [older synonyms cited therein]
-
1991 Aplosmilia semisulcata (Michelin, 1843): Lebanidze, 1991 p. 38–39, Pl. 14, Figs. 3a–b, Text-Fig. 8a–b.
-
2003 Aplosmilia cf. semisulcata Michelin, 1843: Pandey & Fürsich, p. 116–118, Pl. 16 Figs. 7–9.
Dimensions. Great diameter of corallites: 7–12 mm; small diameter of corallites: 6.5–8 mm; in areas of intense budding around 2 mm; septa/corallite: 24 to around 40, in corallites in areas of intense budding around 16; septa/mm: 2–3/2.
Description. Branches of a phaceloid colony; corallites subcircular to elongate in outline; septa developed in 3 to 4 size orders, regularly alternating in length and thickness; lamellar columella preserved deeper in corallum.
Type locality of species. Jurassic of France (Verdun).
Distribution. Jurassic of France, Bathonian–middle Callovian of Iran, Oxfordian of Montenegro, upper Oxfordian of Turkmenistan, upper Oxfordian–Kimmeridgian of Georgia (in Caucasus), lower Kimmeridgian of Portugal and Spain, Tithonian–lower Berriasian of the Czech Republic, upper Berriasian of southern Spain (Andalusia), lower Valanginian of northeastern Switzerland (Vitznau Marl, Wart; this paper).
Material. NMSG Coll. PK–02.10.29h; ?–02.10.35c-C.
Remarks.
Because specimen 02.10.35c-C represents only a small fragment, the assignment to A. semisulcata is provisionally. For further synonyms of the species A. semisulcata, see Paleobiology Database (paleobiodb.org).
Family Axosmiliidae Geyer, 1955
Genus Axosmilia Milne Edwards & Haime, 1848a
Type species. Caryophyllia extinctorium Michelin, 1841, Middle Jurassic (Bajocian) of France (Calvados).
Diagnosis. Solitary, attached by small base or free. Corallite variably conical, elliptical in outline. Costosepta compact, generally free and straight, arranged bilaterally, regularly developed in cycles, granulated laterally. One first cycle septum often fused to columella. Columella lamellar. Distal margin of septa weakly ornamented, showing a zigzag pattern, especially visible in thin septa. Endothecal dissepiments vesicular. Paliform structures and synapticulae absent. Intertrabecular distance ranging between 150 to around 500 µm. Wall made of stereozone and enlarged costae that are fused to each other. Microstructure characterized by mid-septal zigzag line. Epithecal s.l. wall folded.
Axosmilia villersensis (Koby, 1898 )
Fig. 10I
-
*1898 Pleurosmilia villersensis, Koby, 1897: Koby, p. 89–90, Pl. 22, Figs. 2–7.
-
non1936 Pleurosmilia villersensis Koby: Alloiteau, p. 507, Pl. 36, Figs. 1–3.
-
1981 Axosmilia villersensis (Koby, 1898): Turnšek in Turnšek & Mihajlović, p. 22, Pl. 20, Figs. 1–6.
-
1992 Axosmilia villersensis (Koby, 1898 ): Turnšek, p. 164, Fig. 2.
-
nonv1993 Axosmilia villersensis (Koby, 1898): Baron-Szabo, p. 160–161, Pl. 4, Fig. 4.
-
v1996 Axosmilia villersensis (Koby, 1898): Baron-Szabo & Steuber, p. 16–17, Pl. 5, Fig. 6.
-
2021 Miscellosmilia sp.: Löser, et al., p. 34, Figs. 41.1‒2.
Dimensions. Diameter of corallite (d/D): 18 × 20 mm; d/D: 0.9; septa: around 70.
Description. Solitary, corallum incomplete; costosepta straight, developed in 4 to 5 size orders; one septum of S1 connected to lamellar columella.
Type locality of species. Valanginian of Switzerland (Villers).
Distribution. Valanginian of Switzerland (Villers), lower Valanginian of southern Spain and northeastern Switzerland (Vitznau Marl, Wart; this paper), Barremian–lower Aptian of eastern Serbia, lower Aptian of Greece.
Material. NMSG Coll. PK–02.10.10.
Remarks.
Some authors have grouped the species Axosmilia almerai Angelis d’Ossat, 1905, with A. villersensis. However, based on the study of type material it can be said that, in having a corallite diameter of 25 × 30 mm and septa of a little over 48, the species almerai more closely corresponds to A. kobyi. The material from both the Lower Cretaceous of France described in Alloiteau (1936) as Pleurosmilia villersensis and the Aptian of Spain described as A. villersensis in Baron-Szabo (1993) differ from Koby’s species in having 5 complete or nearly complete septal cycles and a corallite that is 12 × 20 mm (d/D: 0.6) in diameter. In having skeletal features closely corresponding to the genus Axosmilia and dimensions of skeletal elements of corallite diameter of 17 × 20 mm (estimated) and septa developed in 4 to 5 size orders (around 80 septa), the material described from the lower Valanginian of southern Spain (Löser et al., 2021) shows very close affinities to A. villersensis.
Availability of data and materials
All specimens illustrated and taxonomically described are stored at the Naturmuseum St, Gallen, Switzerland.
References
Abdel-Gawad, G. L., & Gameil, M. (1995). Cretaceous and Palaeocene coral fauna in Egypt and Greece (2) Palaeontology. Coral Research Bulletin, 4, 6–36. 21 pls.
Alloiteau, J. (1936). Sur le présence, dans le calcaire a Spatangues de la Haute-Marne de, Plesiosmilia villersensis Koby. Bulletin de la Société Géologique de France. 5e série, 6, 507–510.
Alloiteau, J. (1952). Embranchment des Coelentérés. II. Madréporaires post-paléozoïques. In: Piveteau, J. (Ed.), Traité de Paléontologie, Vol. 1. Masson, Paris, pp. 539–684. Retrieved from http://sdrv.ms/RBacJ8.
Alloiteau, J. (1954). Le genre Actinastrea d'Orbigny, 1849 dans le Crétacé supérieur français. Annales Hébert et Haug, 8, 9–104, 10 pls
Alloiteau, J. (1957). Contribution à la systématique des madréporaires fossiles (p. 462). Centre National Recherche Scientifique.
Alloiteau, J. (1958). Monographie des Madréporaires fossiles de Madagascar. Annales Géologiques de Madagascar, 25, 1–218. 38 pls.
Alloiteau, J. (1961). Madréporaires portlandiens de la Querola près d’Alcoy (Espagne). Bulletin de la Société Géologique De France, Paris, 7(11), 288–299.
Alloiteau, J., & Bernier, P. (1970). Amphiphora serannensis nov. gen., nov. sp., nouveau genre de Madréporaire du Jurassique terminal de la bordure méridionale des Cévennes. Bulletin de la Société Géologique de France Paris (7), 11, 925–928. pl. 38 (1969).
Angelis d’Ossat, G. de (1905). Coralli del Cretacico inferiore della Catalogna. Palaeontographica Italica, 11, 169–251. Retrieved from http://archive.org/details/palaeontographia11pisa.
Arkadiev, V. V., & Bugrova, IYu. (1999). Facies of the Cretaceous (Berriasian) deposits from the River Belbek Area (Southwestern Crimea). Facies, 40, 71–80.
Bak, R. P. M., & Elgershuizen, J. H. B. W. (1976). Patterns of oil-sediment rejection in corals. Marine Biology, 37, 105–113.
Baron-Szabo, R. C. (1993). Korallen der höheren Unterkreide (“Urgon”) von Nordspanien (Playa de Laga, Prov. Guernica). Berliner Geowissenschaftliche Abhandlungen (E), 9, 147–181.
Baron-Szabo, R.C. (1997). Zur Korallenfazies der ostalpinen Kreide (Helvetikum, Allgäuer Schrattenkalk; Nördliche Kalkalpen, Brandenberger Gosau), Taxonomie, Paläökologie. Zitteliana, 21, 3–98. Retrieved from http://archive.org/details/zitteliana212319972002baye.
Baron-Szabo, R. C. (2003). Taxonomie und ontogenie von scleractinen Korallen der ostalpinen Oberkreide (Hochmoos- und Grabenbachschichten, Gosau-Gruppe, Santon). Jahrbuch der Geologischen Bundesanstalt, 143, 107–201. Retrieved from http://www.landesmuseum.at/datenbanken/digilit/?litnr=34374.
Baron-Szabo, R. C. (2005). Remarks on the genus Arctangia Wells, 1937, with the re-description of the type species Thecocyathus nathorsti Lindström, 1900 (Anthozoa; Scleractinia) from the Lower Cretaceous of Norway (Spitzbergen). Bulletin of the Biological Society of Washington, 118, 479–482.
Baron-Szabo, R. C. (2008). Corals of the K/T-boundary: Scleractinian corals of the suborders Dendrophylliina, Caryophylliina, Fungiina, Microsolenina, and Stylinina. Zootaxa 1952, 1–244
Baron-Szabo, R. C. (2014). Scleractinian corals from the Cretaceous of the Alps and Northern Dinarides with remarks on related taxa. Abhandlungen der Geologischen Bundesanstalt, 68, 1–287. pls. 1–88, text–figs. 1–22.
Baron-Szabo, R. C. (2016). On the genus Paraclausastrea Zlatarski, 1968 (Scleractinia; Hauterivian–Albian). Jahrbuch der Geologischen Bundesanstalt Wien, 155(1–4), 199–208.
Baron-Szabo, R. C. (2018). Scleractinian corals from the upper Berriasian of central Europe and comparison with contemporaneous coral assemblages. Zootaxa, 4383, 1–98. https://doi.org/10.11646/zootaxa.4383.1.1 12 pls.
Baron-Szabo, R. C. (2021a). Systematic descriptions of the scleractinia family Negoporitidae. In P. A. Selden (Ed.), Treatise on Invertebrate Paleontology, Part F, Revised, Volume 2, Chapter 15, Treatise Online 144 (pp. 1–6). KU Paleontological Institute The University of Kansas.
Baron-Szabo, R. C. (2021b). Upper Barremian–lower Aptian scleractinian corals of central Europe (Schrattenkalk Fm., Helvetic Zone, Austria, Germany, Switzerland). Zootaxa, 4960(1), 1–199. https://doi.org/10.11646/zootaxa.4960.1.
Baron-Szabo, R.C. (2021c). Scleractinian corals of the Albian (uppermost Lower Cretaceous)—overview, revision, evaluation. Proceedings of the Biological Society of Washington, 134(1), 363‒406 and 74 pages (Appendices 1‒8).
Baron-Szabo, R. C., & Fernández-Mendiola, P. A. (1997). Cretaceous scleractinian corals from the Albian of Cabo de Ajo (Cantabria Province, N-Spain). Paläontologische Zeitschrift, 71, 35–50.
Baron-Szabo, R. C., & Furrer, H. (2018). Korallen (Anthozoa). In P. Kürsteiner & C. Klug (Eds.), Fossilien im Alpstein—Kreide und Eozän der Nordostschweiz (pp. 113–143). Appenzeller Verlag.
Baron-Szabo, R.C. & González-León, C.M. (1999). Lower Cretaceous corals and stratigraphy of the Bisbee Group (Cerro de Oro and Lampazos areas), Sonora, Mexico. Cretaceous Research, 20, 465–497. Retrieved from http://www.sciencedirect.com/science/article/pii/S0195667199901593.
Baron-Szabo, R.C. & González-León, C.M. (2003). Late Aptian-Early Albian corals from the Mural Limestone of the Bisbee Group (Tuape and Cerro de Oro areas), Sonora, Mexico. In: Scott, R.W. (Ed.): Cretaceous Stratigraphy and paleoecology, Texas and Mexico: Bob F. Perkins Memorial Volume. Golf Coast Section SEPM Foundation, Special Publications in Geology, 1, CD book, 187–225.
Baron-Szabo, R. C., & Sanders, D. (2020). Scleractinian corals (Anthozoa) from the lower Oligocene (Rupelian) of the Eastern Alps, Austria. Bollettino della Società Paleontologica Italiana, 59, 1–18.
Baron-Szabo, R. C., & Steuber, T. (1996). Korallen und Rudisten aus dem Apt im tertiären Flysch des Parnass-Gebirges bei Delphi-Arachowa (Mittelgriechenland). Berliner Geowissenschaftliche Abhandlungen (E), 18, 3–75.
Baron-Szabo, R. C., Hamedani, A., & Senowbari-Daryan, B. (2003). Scleractinian corals from Lower Cretaceous deposits north of Esfahan (central Iran). Facies, 48, 199–216. pls. 36–39.
Beauvais, M. (1982). Révision systématique des Madréporaires des couches de Gosau (Crétacé supérieur, Autriche). Travaux du Laboratoire de Paléontologie des Invertébrés, 1, 1–256; 2, 1–278; 3, 1–177; 4 (atlas), pls. 1–59; 5 (atlas), figs. 1–131.
Beauvais, L. (1964). Étude stratigraphique et paléontologique des formations à madréporaires du Jurassique supérieur du Jura et de l’Est du Bassin de Paris. Mémoire de la Société géologique de France, 100, 1–287. pls. 1–38.
Beauvais, L. (1973). Upper Jurassic hermatypic corals. In A. Hallam (Ed.), Atlas of palaeobiography. Elsevier Scientific Publishing Company.
Beauvais, L. (1989). Jurassic corals from the circum Pacific area. Memoir of the Association of Australasian Palaeontologists, 8, 291–302.
Beauvais, M., & M’Rabet, A. (1977). Les Madreporaires du Berriasien superieur du Djebel Siouf (Axe Nord-Sud, Tunisie Centrale). Notes du Service Géologique de Tunisie, 43, 103–137. pls. 1–7.
Becker, E. (1876). Die Korallen der Nattheimer Schichten (1). Palaeontographica, 21, 1–60, pls 1–4 and 40–42. Retrieved from http://archive.org/details/palaeontographic21cass.
Bendukidze, N. S. (1961). On the study of the Lower Cretaceous corals from the Crimea. Trudy Geologicheskogo Instituta Akademiya Nauk Gruzinskoy SSR (seriya Geologiya), 12, 5–40. pls. 1–7. in Russian.
Bendukidze, N. S. (1982). Pozdneyurskiye korally rifogennykh otlozheniy Kavkaza i Kryma [Upper Jurassic corals from the reef deposits of the Caucasus and Crimea]. Akademiya Nauk Gruzinskoy SSR, Geologicheskiy Institut Imeni A 1 Dzhanelidze Trudy, 74, 1–166. in Russian.
Bölsche, W. (1871). Die Korallen des unteren Pläners im Sächsischen Elbthale. Palaeontographica, 20, 45–59. Retrieved from http://archive.org/details/palaeontographic20cass.
Bover-Arnal, T., Löser, H., Moreno-Bedmar, J. A., Salas, R., & Strasser, A. (2012). Corals on the slope (Aptian, Maestrat Basin, Spain). Cretaceous Research, 37, 43–64.
Bugrova, IYu. (1990). The facies zonation and scleractinians of the Early Hauterivian reef complex of Bolshoy Balkhan. Cretaceous Research, 11, 229–236. https://doi.org/10.1016/S0195-6671(05)80006-0.
Bugrova, IYu. (1999). New data about the Scleractinia of the lower Barremian sediments of south-west Turkmenistan. In D. L. Stepanov & G. N. Kiselev (Eds.), Voprosy paleontologii (Vol. 11, pp. 33–42). Izd-vo Leningradskogo universiteta. 3 pls. in Russian.
Burger, H. (1985). Palfris-Formation, Öhrli-Formation und Vitznau-Mergel (basale Kreide des Helvetikums) zwischen Reuss und Rhein. Unpublished Ph.D. thesis, University of Zürich, 237 pp.
Burger, H. (1986). Fazielle Entwicklung und paläogeographische Rekonstruktion des helvetischen Schelfs während der untersten Kreide in der Zentral- und Ostschweiz. Eclogae Geologicae Helvetiae, 79, 561–615.
Burger, H., & Strasser, A. (1981). Lithostratigraphische Einheiten der untersten Helvetischen Kreide in der Zentral- und Ostschweiz. Eclogae Geologicae Helvetiae, 74(2), 529–560.
Champagne, T.A.N. (2010). Oligocene coral evolution in Puerto Rico and Antigua: morphometric analysis of Agathiphyllia, Antiguastrea, and Montastraea. MS (Master of Science) thesis, University of Iowa. https://doi.org/10.17077/etd.gl9af2pk.
Coates, A. G., & Jackson, J. B. C. (1987). Clonal growth, algal symbiosis, and reef formation in corals. Paleobiology, 13, 363–378.
Cobb, N. A. (1920). One hundred new names (type species of 100 new genera. Contributions to a Sciences of Nematology, 9, 217–343.
Császár, G., & Turnšek, D. (1996). Vestiges of atoll-like formations in the Lower Cretaceous of the Mecsek Mountains, Hungary. Cretaceous Research, 17, 419–442.
d’Achiardi, A. (1880). Coralli Giurassici dell’Italia Settentrionale: Memoria. Atti della società toscana di scienze naturali residente in Pisa Memorie, 4, 1–78.
d’Orbigny, A. (1849). Prodrôme de Paléontologie stratigraphique universelle des animaux mollusques et rayonnés, Volume 1. Masson, Paris, 394 pp. Retrieved from http://archive.org/details/prodromedepalo01orbiuoft.
d’Orbigny, A. (1850a). Catalogue des espèces fossiles de Mollusques, Bryozoaires, de Polypiers et d’Anamorphozoraires de l’étage néocomien. Revue et Magasin de Zoologie pure et appliquée, sér. 2, t. 2, p. 170–181.
d’Orbigny, A. (1850b). Prodrôme de Paléontologie stratigraphique universelle des animaux mollusques et rayonnés (Vol. 2, p. 428). Masson.
d’Orbigny, A. (1851). Cours élémentaire de Paléontologie (3), Polypiers ou Zoophytes (Vol. 2, pp. 151–189). Masson.
Dacqué, E. (1933). Wirbellose des Jura. Leitfossilien, 7, 1–582. pls 24.
Defrance, M. J. L. (1817). Caryophyllie. In M. J. L. Defrance (Ed.), Dictionnaire des sciences naturelles (Vol. 7, pp. 191–195). L.G. Levrault.
Dietrich, W. O. (1926). Steinkorallen des Malms und der Unterkreide im südlichen Deutsch-Ostafrika. Palaeontographica, 1(Supplement 7), 43–62.
Dimitrijević, D., Raja, B. & Kiessling, W. (2021). Corallite sizes and their link to extinction risk of scleractinian corals across the Triassic-Jurassic boundary. Conference: GSA connects Online. https://doi.org/10.1130/abs/2020AM-357201.
Doweld, A. B. (2014). Starostinia, a new replacement name for Ironella Starostina & Krasnow, 1970 (Anthozoa: Scleractinia: Rhipidogyridae) non Cobb 1920. Zootaxa, 3815(2), 299–300. https://doi.org/10.11646/zootaxa.3815.2.11.
Dryer, S., & Logan, A. (1978). Holocene reefs and sediments of Castle Harbour, Bermuda. Journal of Marine Research, 36, 399–425.
Duncan, P. M. (1870). A monograph of the British fossil corals (2, 2): Corals from the Upper Greensand of Haldon, from the Gault, and the Lower Greensand. Palaeontographical Society Monographs, 23, 45–73.
Duncan, P. M. (1884). A revision of the families and genera of the sclerodermic Zoantharia Edwards et Haime, or Madreporaria (M Rugosa excepted). Journal of the Linnean Society of London, Zoology, 18(104–105), 1–204. https://doi.org/10.1111/j.1096-3642.1884.tb02013.x.
Eguchi, M. (1951). Mesozoic hexacorals from Japan. Science Reports of the Tôhoku Imperial University, Second Series (Geology), 24, 1–96, pls. 1–28. Retrieved from http://jairo.nii.ac.jp/0085/00021417/en.
Eichwald, E. von (1865–69). Lethea rossica ou Paléontologie de la Russie, Vol. 2, Période moyenne. E Schweizerbart, 1288 (in French)
Eliášová, H. (1973). Sous-famille Rhipidogyrinae Koby, 1905 (Hexacorallia) des calcaires de Štramberk (Tithonien, Tchécoslovaquie). Casopis pro Mineralogii a Geologii, 18, 267–287. pls. 1–13.
Eliášová, H. (1976a). Nouvelle famille du sous-ordre Amphiastraeina Alloiteau, 1952 (Hexacorallia, Tithonien de Tchécoslovaquie). Věstnik Ústedního Ústavu Geologického, 51, 177–178. in French.
Eliášová, H. (1976b). Familles Placosmiliidae Alloiteau, 1952 et Misistellidae nov fam (Hexacorallia) des calcaires de Štramberk (Tithonien, Tchecoslovaquie). Casopis pro Mineralogii a Geologii, 21(4), 337–347.
Eliášová, H. (1981). The Tithonian reef of Štramberk Limestone (Czechoslovakia, West Carpathians). Casopis pro Mineralogii a Geologii, 26(2), 113–124.
Eliášová, H. (1990). Coraux des calcaires d’Ernstbrunn (Jurassique supérieur–Crétacé inférieur dans les Carpates externes, zone de Waschberg, Tchécoslovaquie). Casopis pro Mineralogii a Geologii, 35, 113–134. pls. 1–4.
Eliášová, H. (1992a). Rhipidogyridés (Scléractiniaires) du Crétacé de Bohême (Cénomanien supérieur–Turonien inférieur; Turonien supérieur, Tchécoslovaquie). Věstník Českého Geologického Ústavu, 66(3), 163–172. 8 pls. in French.
Eliášová, H. (1992b). Archaeocoeniina, Stylinina, Astraeoina, Meandriina et Siderastraeidae (Scléractiniaires) du Crétacé de Bohême (Cénomanien supérieur–Turonien inférieur; Turonien supérieur, Tchécoslovaquie). Věstník Českého Geologického Ústavu, 67(6), 399–416. 8 pls. in French.
Eliášová, H. (2004). Coraux solitaires (Zoantharia, Microsolenina) du Crétacé de Bohême (Cénomanien supérieur, République Tchèque) [Cretaceous solitary corals (Zoantharia, Microsolenina) from Bohemia (Late Cenomanian, Czech Republic)]. Bulletin of Geosciences, 79(3), 157–166. in French.
Errenst, Ch. (1990). Das korallenführende Kimmeridgium der nordwestlichen iberischen Ketten und angrenzender Gebiete (1). Palaeontographica Serie A, 214, 121–207. pls. 1–12.
Errenst, Ch. (1991). Das korallenführende Kimmeridgium der nordwestlichen iberischen Ketten und angrenzender Gebiete (2). Palaeontographica Serie A, 215, 1–42. pls. 13–21.
Étallon, A. (1858). Etudes paléontologiques sur le Haut-Jura. Rayonnés du Corallien. Mémoires de la Société d’Émulation du Département du Doubs, 3e série, 3, 401–553. Besançon. (in French)
Étallon, A. (1859). Études paléontologiques sur le Haut-Jura. Rayonnés du Corallien. Mémoires de la Société d’Émulation du Département du Doubs, 3e série, 6, 53–260. Retrieved from http://archive.org/details/mmoiresetcomptes03emul.
Felix, J. (1891). Versteinerungen aus der mexicanischen Jura- und Kreide-Formation. Palaeontographica, 37, 140–194. Retrieved from http://archive.org/details/palaeontographic37cass.
Felix, J. (1914). Anthozoa Palaeocretacea. Fossilium Catalogus: Animalia (5–7) (pp. 1–273). W. Junk.
Filkorn, H. F., & Pantoja-Alor, J. (2009). Cretaceous corals from the Huetamo region, Michoacan and Guerrero, southwestern Mexico. Universidad Nacional Autonoma de Mexico, Instituto de Geologia, Boletin, 116, 1–169.
Fischer, P. (1873). Sur le terrain jurassique de Madagascar. Comptes Rendus de l’académie des Sciences, Paris, 76, 111–114.
Föllmi, K. B., Bodin, S., Godet, A., Linder, P., & Van de Schootbrugge, B. (2007). Unlocking paleo-environmental information from Early Cretaceous shelf sediments in the Helvetic Alps: Stratigraphy is the key! Swiss Journal of Geosciences, 100(3), 349–369. https://doi.org/10.1007/s00015-007-1236-y.
Frech, F. (1890). Die Korallenfauna Der Trias. Palaeontographica, 37, 1–116.
Fromentel, E. de (1856). Note sur les polypiers fossiles de étage portiandien de la Haute-Saône. Bulletin de la Société géologique de France, Série 2, 13, 851–865. Retrieved from http://books.google.com/books?id=36obAAAAMAAJ.
Fromentel, E. de (1857). Description des Polypiers fossiles: Étage Nèocomien. Bulletin de la Société des Sciences Historiques et Naturelles de l’Yonne, 78 pp. Retrieved from http://books.google.com/books?id=EL8oAQAAMAAJ.
Fromentel, E. de (1861). Introduction à l’étude des Polypiers fossiles. Mémoires de la Société d’Émulation du Département du Doubs, 5, 1–357. Retrieved from http://archive.org/details/mmoiresetcomptes05emul.
Fromentel, E. de (1884). Zoophytes, terrains crétacés (14). In A. de d’Orbigny (Ed.), Paléontologie Française (Vol. 8, pp. 529–560). Masson. pls. 145–156.
Fromentel, E. de (1887). Zoophytes, terrains crétacés (16). In A. d’Orbigny (Ed.), Paléontologie Française (Vol. 8, pp. 609–624). Masson. pls. 181–192.
Funk, H. (1999). Kreide des Säntis. Exkursion H. 69. Jahrestagung der Paläontologischen Gesellschaft 20.9.–26.9.1999 Zürich. Terra Nostra, 99(9), 113–127.
Garberoglio, R. M., Löser, H., & Lazo, D. G. (2020). Lower Cretaceous corals from the Agrio Formation, Neuquén Basin, west-central Argentina: Family Actinastraeidae. Cretaceous Research, 114, 18. https://doi.org/10.1016/j.cretres2020.104503.
Garberoglio, R.M., Löser, H. & Lazo, D.G. (2021). Lower Cretaceous corals from the Agrio Formation, Neuquén Basin, west-central Argentina: Family Columastraeidae. Cretaceous Research, 124(suppl. 7), 104817, 1–19. https://doi.org/10.1016/j.cretres.2021.104817.
Geyer, O. (1954). Die oberjurassische Korallen-Fauna von Württemberg. Palaeontographica Abteilung A: Paläozoologie, Stratigraphie, 104, 121–220. pls. 9–16.
Geyer, O. F. (1955). Beiträge zur Korallenfauna des Štramberger Tithon. Paläontologische Zeitschrift, 29, 177–216. https://doi.org/10.1007/BF03041799 pls. 22–26.
Geyer, O. F., & Rosendahl, S. (1985). Stromatoporen, Korallen und Nerineen aus oberjurassischen und unterkretazischen Schichten des Präbetikums von Cazorla (Provinz Jaen, Spanien) [Upper Jurassic and Lower Cretaceous stromatoporoids, corals and nerineids from the Prebetic of Cazorla (Jaen, Spain)]. Arbeiten aus dem Institut für Geologie und Paläontologie an der Universität Stuttgart, 82, 161–179.
Ginsburg, R. N. (1972). South Florida Carbonate Sediments. Sedimenta, 2, 1–71.
Goldfuss, G.A. (1826–1829). Petrefacta Germaniae. Volumes 1–2. Arnz & Co., Düsseldorf, pp. 1–164. Retrieved from http://sdrv.ms/WzHwEL.
Hackemesser, M. (1936). Eine kretazische Korallenfauna aus Mittel-Griechenland und ihre paläobiologischen Beziehungen. Palaeontographica, 84, 1–97.
Haefeli, Ch., Maync, W., Oertli, H. J., & Rutsch, R. F. (1965). Die Typus-Profile des Valanginien und Hauterivien. Bulletin der Vereinigung Schweiz Petroleum-Geologen und Ingenieure, 31(81), 41–75.
Idakieva, V. (2007). Taxonomy of scleractinian corals from the Barremian-Lower Aptian of central north Bulgaria (Lovech Urgonian Group). Godishnik na Sofiyskiya Universitet Sv. Kliment Okhridski, Geologo-Geografski Fakultet, Geologiya, 100, 29–66.
Imlay, R. W. (1940). Neocomian fauna of northern Mexico. Geological Society of America, Bulletin, 51, 117–190.
Jaccard, A. (1893). Contributions à la géologie du Jura. Bulletin de la Société des Sciences Naturelles de Neuchâtel, 21, 51–87. Neuchâtel.
Jordi, H.A. (2012). Blatt 1188 Eggiwil. Erläuterungen 75. Geologischer Atlas der Schweiz, 1:25000, Bundesamt für Landestopografie, pp. 1‒72, 2 pls.
Karakasch, N. I. (1907). Le crétacé inférieur de la Crimée et sa fauna. Trudy Imperatorskogo St. Petersburgskago Obshchestva Estestvoispytateley, 32, 1–484.
Kashiwagi, K., Yamagiwa, N., Yao, A., Ezaki, Y., Sakaori, Y., & Shoji, Y. (2002). Late Jurassic cnidarian and poriferan fossils from the Torinosu-type limestones in the Kurosegawa Terrane, western Kii Peninsula, southwest Japan and their geological significance. Paleontological Society of Japan, Tokyo, 72, 5–16.
Kaya, O., Wiedmann, J., Kozur, H., Özdemir, Ü., Özer, S., & Beauvais, L. (1987). A new discovery of the Lower Cretaceous in Istanbul-Turkey. Bulletin of the Mineral Research and Exploration, 107, 106–111.
Kitchin, F. L. (1908). The invertebrate fauna and palaeontological relations of the Uitenhage series. Annals of the South African Museum, 7, 21–250.
Koch, C. L., & Dunker, W. B. R. H. (1837). Beitrāge zur Kenntniss des Norddeutschen Oolithgebildes und dessen Versteinerungen. Volume 4, 1–64; Oehme und Mülle, Braunschweig.
Koby, F. (1880). Monographie des polypiers jurassiques de la Suisse (4). Mémoires de la Société Paléontologique Suisse (= Abhandlungen der Schweizerischen Paläontologischen GeselIschaft), 7, 1–60. pls. 1–12 in French.
Koby, F. (1881). Monographie des polypiers jurassiques de la Suisse (4). Mémoires de la Société Paléontologique Suisse (= Abhandlungen der Schweizerischen Paläontologischen GeselIschaft), 8, 61–108, pls. 13–30. in French
Koby, F. (1884). Monographie des polypiers jurassiques de la Suisse (4). Mémoires de la Société Paléontologique Suisse (= Abhandlungen der Schweizerischen Paläontologischen GeselIschaft), 11, 149–212, pls. 43–63. (in French) (http://sdrv.ms/RBjoNA).
Koby, F. (1885). Monographie des polypiers jurassiques de la Suisse (4). Mémoires de la Société Paléontologique Suisse (= Abhandlungen der Schweizerischen Paläontologischen GeselIschaft), 12, 213–304. pls. 64–81. in French.
Koby, F. (1887). Monographie des polypiers jurassiques de la Suisse (7). Mémoires de la Société Paléontologique Suisse (= Abhandlungen der Schweizerischen Paläontologischen GeselIschaft, 14, 353–400, pls. 99–108. (in French) Retrieved from http://books.google.com/books?id=xt8QAAAAIAAJ.
Koby, F. (1896). Monographie des polypiers crétacés de la Suisse (1). Mémoires de la Société Paléontologique Suisse (= Abhandlungen der Schweizerischen Paläontologischen GeselIschaft), 22, 1–28, pls. 1–8. (in French) Retrieved from http://sdrv.ms/RBjB3y.
Koby, F. (1897). Monographie des polypiers crétacés de la Suisse (2). Mémoires de la Société Paléontologique Suisse (= Abhandlungen der Schweizerischen Paläontologischen GeselIschaft), 23, 29–62, pls. 9–16. (in French) Retrieved from http://sdrv.ms/RBjGUJ.
Koby, F. (1898). Monographie des polypiers crétacés de la Suisse (2). Mémoires de la Société Paléontologique Suisse (= Abhandlungen der Schweizerischen Paläontologischen GeselIschaft), 24, 63–100. pls. 17–22 in French.
Koby, F. (1905). Description de la faune jurassique du Portugal. Polypiers du Jurassique supérieur. Commission du Service Géologique du Portugal, 89–164, pls. 1–30. [in French] Retrieved from http://hdl.handle.net/2027/uc1.31822023452386).
Kołodziej, B. (1995). Microstructure and taxonomy of Amphiastraeina (Scleractinia). Annales Societatis Geologorum Poloniae, 65, 1–17.
Kołodziej, B. (2003). Scleractinian corals of suborders Pachythecaliina and Rhipidogyrina: discussion on similarities and description of species from Štramberk-type limestones, Polish Outer Carpathians. Annales Societatis Geologorum Poloniae, 73, 193–217. Retrieved from http://www.asgp.pl/73_3_193.
Kołodziej, B. (2015). Corals of the Štramberk-type limestone from Poland: Taxonomic and palaeoecological aspects. Neues Jahrbuch für Geologie und Paläontologie Abhandlungen, 276(2), 181–199.
Kołodziej, B., & Marian, V. A. (2021). Simplified, wall- based morphology of a new Aptian coral and discussion of contrasting opinions on the taxonomy of similar corals. Neues Jahrbuch für Geologie und Paläontologie Abhandlungen, 300(2), 201–213.
Krasnov, E.V. & Starostina, E.A. (1970). Late Jurassic scleractinians of northern Caucasus. In: Sokolov, B.S. (Ed.): Mezozoyskie Korally SSSR (Trudy 2 Vsesoyuznogo simpoziuma po izucheniyu iskopaemykh korallov SSSR, 4) [Proceedings of the 2-Union Symposium on the study of fossil corals of the USSR], 75–80, Moskva (Nauka). (in Russian)
Kürsteiner, P., & Klug, C. (2018). Fossilien im Alpstein – Kreide und Eozän der Nordostschweiz (p. 376). Appenzeller Verlag.
Kuzmicheva, E.I. (1966). Stratigraphic and facial distribution of hexacorals (Scleractinia) in the Neocomian of the Mountain Crimea. Prirodnye I trudovye resursy levoberezhnoy Ukrainy i ikh isolzovanie, 58–63 (Moskva, Nedra) [in Russian]
Kuzmicheva, E. I. (1967). Morphology of Early Cretaceous scleractiniae. Paleontologichesky Zhurnal, 4, 48–55. in Russian.
Kuzmicheva, E. I. (1972). Novye vidy rannemelovykh skleraktiniy gornogo Kryma [New species of Early Cretaceous scleractinians from the Mountain Crimea] (pp. 106–110). Nauka. in Russian.
Kuzmicheva, E. I. (1980). Corals. In V. Chernov, B. Yanin, & M. Golovinova (Eds.), Urgonskie Otlozheniya Sovetskikh Karpat (pp. 90–108). Nauka. in Russian.
Kuzmicheva, E. I. (1985). Cretaceous and Paleogene corals of the Ukraine. Moscow University Geology Bulletin, 40(5), 30–35.
Kuzmicheva, E. I. (2002). Skeletal morphology, systematics and evolution of Scleractinia. Trudy Paleontologicheskogo Instituta, 286, 1–211. in Russian.
Kuzmicheva, E. I., & Aliev, O. B. (1988). Corals. In A. A. Ali-Zade, G. A. Aliev, M. M. Aliev, H. Aliujulla, & A. G. Halilov (Eds.), Cretaceous fauna of Azerbaijan (pp. 153–184). Akademia Nauk Azerbaidjana SSR. in Russian.
Kuzmicheva, E. I., & Shalya, A. A. (1962). Biogenic formations in the Neocomian deposits of the Central Crimea. Izvestiya vysshikh uchebnykh zavedeny, geologiya i razvedka, 12, 29–34.
Lamouroux, J.U.F. (1821). Exposition méthodique des genres de l'ordre des polypiers. Agasse, Paris, 115 pp. pls. 1–84. Retrieved from http://archive.org/details/expositionmtho00lamo.
Laternser, R. (2001). Oberjurassische Korallenriffe von Nordfrankreich (Lothringen) und Südwestdeutschland. PhD Thesis, University of Stuttgart, 298 pp.
Lathuilière, B. (1996). Itinéraires astogéniques chez des coraux simples et coloniaux montlivaltiides du Bajocien de France. Geobios 29, 577–603. Retrieved from http://www.sciencedirect.com/science/article/pii/S001669959680026X.
Lathuilière, B., Baron-Szabo, R.C., Charbonnier, S. & Pacaud, J.-M. (2020). The Mesozoic scleractinian genus Adelocoenia (Stylinidae) and its Jurassic species. Carnets de Geologie, 20 (19), 367–406, figs. 1–36. https://doi.org/10.2110/carnets.2020.2019.
Lathuilière, B., & Gill, G. (1998). Dendraraea corail scléractiniaire branchu jurassique: Structure, systématique, écologie. Palaeontographica, 248, 145–162.
Lauxmann, U. (1991). Revision der oberjurassischen Korallen von Württemberg (SW-Deutschland), exclusive Fungiina. Palaeontographica Abteilung A: Paläozoologie, Stratigraphie, 219, 107–175. pls. 1–7.
Lebanidze, Z. M. (1991). Pozdiejurskie korraly zapadnoj gruzii (Abkhazja). Trudy Geologicheskogo Instituta Akademiya Nauk Gruzinskoy SSR (Seriya Geologiya), 105, 1–65. in Russian.
Lefeld, J. (1968). Stratigraphy and paleogeography of the High-Tatric Lower Cretaceous in the Tatra Mountains. Studia Geologica Polonica, 24, 115. pls. 18 in Polish.
Liao, W. (1982). Mesozoic scleractinian corals from Xizang (Tibet) (pp. 151–183). Beijing: Science Press. in Chinese with English summary.
Liao, W.-H., & Xia, J.-B. (1985). Upper Jurassic and Lower Cretaceous Scleractinia from Bangoin district of northern Xizang (Tibet). Memoirs of the Nanjing Institute of Geology and Palaeontology, 21, 119–174. in Chinese with English summary.
Liao, W.-H., & Xia, J.-B. (1994). Mesozoic and Cenozoic scleractinian corals from Xizang. Palaeontologia Sinica, 184(31), 1–252.
Linnaeus, C. von (1758). Systema naturæ per regna tria naturæ, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Tomus I, pp. 824, Holmiæ [=Stockholm] (Laurentius Salvius). (in Latin)
Logan, A. (1988). Sediment-shifting capability in the recent solitary coral Scolymia cubensis (Milne-Edwards and Haime) from Bermuda. Bulletin of Marine Science, 43, 241–248.
Loriol, P. de (1868). Monographie des couches de l’Étage Valanginien des carrièrs d’Arzier (Vaud). In: Pictet, F.-J. (ed), Matériaux pour la paleontology Suisse: ou recueil des monographies sur les fossils du Jura et des Alpes (4), 2. H. Georg, Bale and Geneva, pp. 5‒110, 9 pls.
Löser, H. (1989). Die Korallen der Sächsischen Oberkreide, Teil 1. Abhandlungen des Staatlichen Museums für Mineralogie und Geologie Dresden, 36, 88–154. 183–186, 209–215, pls. 21–27.
Löser, H. (1998). Die Korallen der Sächsichen Oberkreide—eine Zwischenbilanz und Bermerkungen zu Korallenfaunen des Cenomans. Abhandlungen des Staatlichen Museums für Mineralogie und Geologie zu Dresden, 43(44), 173–187.
Löser, H. (2001). Le site de Valliere (departement de l’Aube, France): resultats preliminaire sur des coraux de l’Hauterivien inferieur (Cretace). Bulletin annuelle de l'Association geologique de l'Aube, 22, 39–53 (Sainte Savine; Association geologique auboise).
Löser, H. (2008). Early Cretaceous coral faunas from East Africa (Tanzania, Kenya; Late Valanginian–Aptian) and revision of the Dietrich collection (Berlin, Germany). Palaeontographica (Abteilung A), 285(1–3), 23–75.
Löser, H. (2012). Revision of Actinastrea, the most common Cretaceous coral genus. Paläontologische Zeitschrift, 86, 15–22
Löser, H., Arias, C., & Vilas, L. (2015). Aptian-Albian coral faunas from the Sierra del Carche (Prebetic, Murcia, Southern Spain). Spanish Journal of Palaeontology, 30(1), 43–63.
Löser, H., Arias, C., & Vilas, L. (2019). Upper Valanginian to Lower Hauterivian coral faunas from the Sierra Larga (Prebetic zone, SE Spain). Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen, 292(3), 259–290.
Löser, H., Nieto, L.M., Castro, J.M & Reolid, M. (2021). A Lower Valanginian coral faunas from the South Iberian Palaeomargin (Internal Prebetic, SE Spain). Palaeontologia Electronica, 21(1), 1–57 (a06). https://doi.org/10.26879/1030.
Masse, J.-P., Morycowa, E., & Fenerci-Masse, M. (2009). Valanginian-Hauterivian scleractinian coral communities from the Marseille region (SE France). Cretaceous Research, 30, 178–192.
Michelin, H. (1841). Iconographie zoophytologique. Description par localités et terrains des polypiers fossiles de France (Vol. 2, pp. 18–72). Paris: Bertrand.
Michelin, H. (1843). Iconographie zoophytologique. Description par localités et terrains des polypiers fossiles de France (Vol. 3, pp. 73–104). Bertrand.
Michelin, H. (1844). Iconographie zoophytologique. Description par localités et terrains des polypiers fossiles de France (Vol. 4, pp. 105–144). Bertrand.
Michelin, H. (1847). Iconographie zoophytologique. Description par localités et terrains des polypiers fossiles de France (Vol. 7, pp. 249–328). Bertrand.
Milaschewitsch, C. (1876). Die Korallen der Nattheimer Schichten (2). Palaeontographica, 21, 62–123. pls 43–51.
Milne Edwards, H., & Haime, J. (1848a). Note sur la classification de la deuxième tribu de la famille des astréides. Comptes Rendus de l’académie des Sciences, 27, 490–497.
Milne Edwards, H., & Haime, J. (1848b). Recherches sur les polypiers (4). Monographie des astréides (1) Eusmiliens. Annales des sciences naturelles, série 3. Zoologie, 10, 209–320. pls. 5–9.
Milne Edwards, H. & Haime, J. (1850). A monograph of the British fossil corals (1). Tertiary and Cretaceous. Monographs of the Palaeontographical Society, 3, i–lxxxv, 1–71, pls. 1–11.
Milne Edwards, H., & Haime, J. (1851). A monograph of the British fossil corals. Corals from the oolitic formations. Monographs of the Palaeontographical Society, 5, 73–146. pls. 12–30.
Milne Edwards, H. & Haime, J. (1857). Histoire naturelle des Coralliaires ou polypes proprement dits. Volumes 1 and 2. Librairie Encyclopédique de Roret, Paris, 633 pp. and atlas. Retrieved from http://books.google.com/books?id=W48-AAAAcAAJ&source=gbs_navlinks_s.
Morales, C., Gardin, S., Schnyder, J., Spangenberg, J., Arnaud-Vanneau, A., Arnaud, H., Adatte, T., & Föllmi, K. B. (2013). Berriasian and early Valanginian environmental change along a transect from the Jura platform to the Vocontian Basin. Sedimentology, 60(1), 36–63.
Morycowa, E. (1964). Hexacorallia des couches de Grodziszcze (Néocomien, Carpathes). Acta Palaeontologica Polonica, 9, 3–114, pls. 1–33. (in French) Retrieved from http://www.app.pan.pl/article/item/app09-003.html.
Morycowa, E. (1971). Hexacorallia et Octocorallia du Crétacé inférieur de Rarau (Carpathes orientales roumaines). Acta Palaeontologica Polonica, 16, 3–149. (in French). Retrieved from http://www.app.pan.pl/article/item/app16-003.html.
Morycowa, E. (2012). Corals from the Tithonian carbonate complex in the Dabrowa Tarnowska-Szczucin area (Polish Carpathian Foreland). Annales Societatis Geologorum Poloniae, 82, 1–38.
Morycowa, E. & Masse, J.-P. (1998). Les scléractiniaires du Barrémien–Aptien inférieur de Provence (SE de la France) [The Barremian–Early Aptian Scleractinia from Provence (SE France)]. Géobios, 31 (6), 725–766 (in French). Retrieved from http://www.sciencedirect.com/science/article/pii/S0016699598801071.
Morycowa, E. & Marcopoulou-Diacantoni, A. (2002). Albian corals from the subpelagonian zone of central Greece (Agrostylia, Parnassos region). Annales Societatis Geologorum Poloniae, 72, 1–65. Retrieved from http://www.asgp.pl/72_1_001.
Morycowa, E. & Roniewicz, E. (1990). Revision of the genus Cladophyllia and description of Apocladophyllia gen.n. (Cladophylliidae fam.n., Scleractinia). Acta Palaeontologica Polonica, 35, 165–190, pls. 15–22. Retrieved from http://www.app.pan.pl/article/item/app35-165.html.
Morycowa, E., & Decrouez, D. (2006). Early Aptian scleractinian corals from the Upper Schrattenkalk of Hergiswil (Lucerne region, Helvetic Zone of central Switzerland). Revue de Paléobiologie, 25(2), 791–838.
Morycowa, E., & Masse, J.-P. (2009). Lower Cretaceous Microsolenina (Scleractinia) from Provence (southern France). Annales Societatis Geologorum Poloniae, 79, 97–140.
Morycowa, E., & Roniewicz, E. (2016). Microstructural evidence of the stylophyllid affinity of the genus Cyathophora (Scleractinia, Mesozoic). Annales Societatis Geologorum Poloniae, 86, 1–16. https://doi.org/10.14241/asgp.2015.023.
Morycowa, M., & Mišik, M. (2005). Upper Jurrasic shallow-water scleractinian corals from the Pieniny Klippen Belt (Western Carpathians/Slovakia). Geologica Carpathica, 56(5), 415–432.
Ogilvie, M.M. (1897). Die Korrallen der Stramberger Schichten. Palaeontographica, 7A, 73–282. Retrieved from http://sdrv.ms/QWbjTX.
Oppenheim, P. (1930). Die Anthozoen der Gosauschichten in den Ostalpen. Oppenheim (privately published), Berlin-Lichterfelde, 604 pp.
Pandey, D. K., & Fürsich, F. T. (2003). Jurassic corals of east-central Iran. Beringeria, 32, 1–138.
Pandey, D.K., Fürsich, F.T., Baron-Szabo, R.C. & Wilmsen, M. (2007). Lower Cretaceous corals from the Koppeh Dagh, NE-Iran. Zitteliana A, 48–49, 13–37. Retrieved from http://epub.ub.uni-muenchen.de/11965/.
Papoyan, A. S. (1982). Biostratigrafi korallov iz pozdnejurskogo Neokomskogo kompleksa zangesura. Izvestiya AN Armyanskoy SSR, Nauki o Zemle, Erevan, 35(2), 65–67.
Papoyan, A. S. (1989). A biostratigraphic review of the Kaphan anticlinorium Upper Jurassic-Lower Cretaceous deposits by the coralline fauna. Izvestiya AN Armyanskoy SSR, Nauki o Zemle, Erevan, 42(2), 3–9. in Russian.
Pfister, T. (1980). Systematische und paläoökologische Untersuchungen an oligozänen Korallen der Umgebung von San Luca (Provinz Vicenza, Norditalien). Schweizerische Paläontologische Abhandlungen (mémoires Suisses de Paléontologie), 103, 1–121.
Phillips, J. (1829). Illustrations of the Geology of Yorkshire; or, a Description of the Strata and Organic Remains of Yorkshire Coast. York. Thomas Wilson and Sons, pp. 192, 25 pls. Retrieved from http://archive.org/details/illustrationsofg00philrich.
Pratz, E. (1882–1883). Über die verwandtschaftlichen Beziehungen einiger Korallengattungen mit hauptsächlicher Berücksichtigung ihrer Septalstructur. Palaeontographica, 29, 81–124. [Cassel (=Kassel)]. Retrieved from http://sdrv.ms/TcfboO.
Prever, P.L. (1909). Anthozoa. In: Parona, C.F. (Ed.), La Fauna Coralligena del Cretaceo dei Monti d’Ocre nell’Abruzzo Aquilano. Springer, Roma, pp. 51–147. Roma. [in Italian]
Prinz, P. (1991). Mesozoische Korallen aus Nordchile. Palaeontographica Abteilung A: Paläozoologie, Stratigraphie, 216, 147–209.
Quenstedt, F.A. (1843). Das Flözgebirge Würtembergs. Mit besonderer Rücksicht auf den Jura. Tübingen: H. Laupp, XXXX pp. Retrieved from http://books.google.com/books?id=3fYTAAAAQAAJ.
Quenstedt, F.A. (1852). Handbuch der Petrefaktenkunde. H. Laupp, Tübingen, 796 pp.
Quenstedt, F.A. (1857). Der Jura (Teil 3). H. Laup & Sieber, Tübingen, pp. 369–576. Retrieved from http://books.google.com/books?id=jCI-AAAAcAAJ.
Quenstedt, F. A. (1879). Röhren- und Sternkorallen (Teil 1) (pp. 1–624). Fues’s Verlag.
Reig Oriol, J.M. (1994). Madreporarios cretácicos de Cataluña. José M Reig Oriol (privately published), Barcelona, 60 pp.
Reyeros de Castillo, M.M. (1983). Corales de algunas formaciones cretacicas del estado de Oaxaca. Paleontologia Mexicana, 47, 1–67. Retrieved from http://www.ojs-igl.unam.mx/index.php/Paleontologia/article/view/72.
Ricci, C., Lathuilière, B., & Rusciadelli, G. (2018). Coral communities, zonation and paleoecology of an Upper Jurassic reef complex (Ellipsactinia Limestones, central Apennines, Italy). Rivista Italiana di Paleontologia e Stratigrafia, 124(3), 433–508.
Roniewicz, E. (1976). Les scléractiniaires du Jurassique supérieur de la Dobrogea centrale Roumanie. Palaeontologica Polonica, 34, 17–121, pls. 1–34. (in French) Retrieved from http://www.palaeontologia.pan.pl/Archive/1976-34.pdf.
Roniewicz E. (1989). Triassic scleractinian corals of the Zlambach Beds, northern Calcareous Alps, Austria. Denkschriften Österreichische Akademie der Wissenschaften, Mathematisch-Naturwissenschaftliche Klasse 126, 1–144.
Roniewicz, E. (2008). Kimmeridgian–Valanginian reef corals from the Moesian Platform from Bulgaria. Annales Societatis Geologorum Poloniae, 78, 91–134. Retrieved from http://www.asgp.pl/78_2_091_134.
Roniewicz, E., & Morycowa, E. (1993). Evolution of the Scleractinia in the light of microstructural data. Courier Forschungsinstitut Senckenberg, 164, 233–240.
Rosendahl, S. (1985). Die oberjurassische Korallenfazies von Algarve (Südportugal). Arbeiten aus dem Institut für Geologie und Paläontologie der Universität Stuttgart Neue Folge, 82, 1–125. pls. 1–11.
Sala, P., Pfiffner, A. O., & Frehner, M. (2014). The Alpstein in three dimensions: Fold-and-thrust belt visualization in the Helvetic zone, eastern Switzerland. Swiss Journal of Geoscience. https://doi.org/10.1007/s00015-014-0168-6.
Sanders, D. G., & Baron-Szabo, R. C. (2005). Scleractinian assemblages under sediment input: Their characteristics and relation to the nutrient input concept. Palaeogeography, Palaeoclimatology, Palaeoecology, 216, 139–181. https://doi.org/10.1016/j.palaeo.2204.10.008.
Sandy, M. R. (1990). Early Cretaceous brachiopods from Mexico and their paleobiogeographic significance. Journal of Paleontology, 64(6), 942–956.
Santodomingo, N. (2014). Miocene reef-coral diversity of Indonesia: unlocking the murky origins of the Coral Triangle. Utrecht studies in earth sciences, 63, 1–340
Schöllhorn, E. (1998). Geologie und Paläontologie des Oberapt im Becken von Organyà (Nordspanien). Coral Research Bulletin, 6, 1–139.
Scholz, H. (1979). Paläontologie, Aufbau und Verbreitung der Bioherme und Biostrome im Allgäuer Schrattenkalk. Unplublished PhD thesis, Technische Universität München, München, pp. 133
Scholz, H. (1984). Paläontologie, Aufbau und Verbreitung der Bioherme und Biostrome im Allgäuer Schrattenkalk (Helvetikum, Unterkreide). Jahrbuch der Geologischen Bundesanstalt Wien, 127(3), 471–499.
Scott, R.W. (1984). Significant fossils of the Knowles Limestone, Lower Cretaceous, Texas. In: Ventress, W.P.S. et al. (Eds.): The Jurassic of the Gulf Rim: Gulf Coast Section, SEPM Foundation, Proceedings Third Annual Research Conference, 333–346.
Shishlov, S. B., Dubkova, K. A., Arkadiev, V. V., Bugrova, I. Y., Bugrova, E. M., Trikolidi, F. A., & Zakrevskaya, E. Y. (2020). Cretaceous and Paleogene of the Bodrak River Basin (South-Western Crimea) (p. 9). Saint-Petersburg University Centre for Geology LLC, Saint-Petersburg State University, Institute of Earth Sciences. in Russian.
Sikharulidze, G. Y. (1972). The new genus Paretallonia (Hexacorallia) from Lower Cretaceous sediments in western Georgia. Soobshcheniya Akademii Nauk Gruzinskoy SSR, 68, 641–644.
Sikharulidze, G. Y. (1977). Lower Cretaceous hexacorals from Georgia. Paleontologiya i Stratigraftya Mezozoyskikh Odozheniy Gruzii, 3, 66–109. pls. 1–18 in Russian.
Sikharulidze, G. Y. (1979). Albiskie korally sela Tshanari [Albian corals from the village Tskhanari]. Trudy Geologicheskogo Instituta Akademiya Nauk Gruzinskoy SSR (seriya Geologiya), 63, 1–49. pls. 1–26 in Russian.
Sikharulidze, G. Y. (1985). Geksakorally urgonskoy fatsii dzirul’skogo massiva i ego severnogo obramleniya [Hexacorals from the Urgonian facies of the Dzirul Massif and its southern frame]. Trudy Geologicheskogo Instituta Akademiya Nauk Gruzinskoy SSR (Seriya Geologiya), 88, 1–110. pls. 1–31 in Russian.
Solomko, E. (1888). Die Jura- und Kreidekorallen der Krim. Verhandlungen der Russisch-Kaiserlichen Mineralogischen Gesellschaft zu St. Petersburg (2), 24, 67–232, pls. 1–8. Retrieved from http://archive.org/details/diejuraundkreid00sologoog.
Stafford-Smith, M. G. (1993). Sediment-rejection efficiency of 22 species of Australian scleractinian corals. Marine Biology, 115, 229–243.
Tomás, S., Löser, H., & Salas Roig, R. (2008). Low-light and nutrient-rich coral assemblages in an Upper Aptian carbonate platform of the southern Maestrat Basin (Iberian Chain, eastern Spain). Cretaceous Research, 29, 509–534.
Toula, F. (1889). Geologische Untersuchungen im centralen Balkan. Denkschriften der Kaiserlichen Akademie der Wissenschaften, Mathematisch-Naturwissenschaftliche Classe, 55, 1–108. Retrieved from http://www.landesmuseum.at/datenbanken/digilit/?litnr=30464.
Trautschold, H. (1886). Le Néocomien de Sably en Crimée. Trudy Imperatorskogo St.-Petersburgago Obshchestva Estestvoispytateley, 15, 119–129.
Turnšek, D. (1972). Upper Jurassic corals of southern Slovenia. Razprave Slovenska Akademija Znanosti in Umetnosti (4), 15, 147–265. pls. 1–37.
Turnšek, D. (1992). Tethyan Cretaceous corals in Yugoslavia. Österreichische Akademie der Wissenschaften, Schriftenreihe der Erdwissenschaftlichen Kommissionen, 9, 155–170, Wien.
Turnšek, D. (1997). Mesozoic corals of Slovenia (p. 512). Znanstvenoraziskovalni Center SAZU.
Turnšek, D., & Buser, S. (1974). The Lower Cretaceous corals, hydrozoans and chaetetids of Banjska Planota and Trnovski Gozd. Razprave Slovenska Akademija Znanosti in Umetnosti (4), 17, 85–124. pls. 1–16.
Turnšek, D., & Buser, S. (1976). Knidarijska favna iz senonijske breče na Banjški planoti [Cnidarian fauna from the Senonian breccia of Banjska Planota (NW Yugoslavia)]. Razprave Slovenska Akademija Znanosti in Umetnosti (4), 19, 39–88. pls. 1–25.
Turnšek, D., & Mihajlović, M. (1981). Lower Cretaceous cnidarians from eastern Serbia. Razprave Slovenska Akademija Znanosti in Umetnosti (4), 23, 1–54. pls. 1–50.
Turnšek, D., Pleničar, M., & Šribar, L. (1992). Lower Cretaceous fauna from Slovenski Vrh near Koevje (South Slovenia). Razprave Slovenska Akademija Znanosti in Umetnosti (4), 33, 205–257.
Turnšek, D., LeMone, D.V. & Scott, R.W. (2003). Tethyan Albian corals, Cerro de Cristo Rey Uplift, Chihuahua and New Mexico. In: Scott, R.W. (Ed.). Cretaceous Stratigraphy and paleoecology, Texas and Mexico: Bob F. Perkins Memorial Volume. Golf Coast Section SEPM Foundation, Special Publications in Geology, 1, CD book, 147–185.
Tyson, R. V., & Funnell, B. M. (1987). European Cretaceous shorelines, stage by stage. Palaeogeography, Palaeoclimatology, Palaeoecology, 59, 69–91.
Vaughan, T.W. & Wells, J.W. (1943). Revision of the suborders, families and genera of the Scleractinia. Geological Society of America, Special Paper, 44, 1–363 [Boulder, Colorado]
Von der Osten, E. (1957). A fauna from the Lower Cretaceous Barranquín formation of Venezuela. Journal of Paleontology, 31, 571–590.
Wells, J. W. (1932). Corals of the trinity group of the comanchean of central texas. Journal of Paleontology, 6, 225–256.
Wells, J. W. (1933). Corals of the cretaceous of the Atlantic and Gulf coastal plains and western interior of the United States. Bulletins of American Paleontology, 18(67), 1–207.
Wells, J. W. (1944). Cretaceous, Tertiary and Recent corals, a sponge and an alga from Venezuela. Journal of Paleontology, 18, 429–447.
Wells, J. W. (1946). Some Jurassic and Cretaceous corals from Northern Mexico. Journal of Paleontology, 20(1), 1–7.
Wells, J. W. (1956). Part F, Coelenterata. In R. C. Moore (Ed.), Treatise on invertebrate paleontology (pp. F328–F444). University of Kansas Press.
Wilmsen, M. (1996). Flecken-Riffe in den Kalken der “Formación de Altamira” (Cenoman, Cobreces/Toñanes-Gebiet, Prov. Kantabrien, Nord-Spanien): Stratigraphische Position, fazielle Rahmenbedingungen und Sequenzstratigraphie. Berliner Geowissenschaftliche Abhandlungen, Reihe E, 18, 353–373.
Zaman, S., & Lathuilière, B. (2014). A lectotype for Cyathophora richardi Michelin 1843. Zootaxa, 3795(2), 198–200.
Acknowledgements
Our thanks and gratitude go to Dennis Opresko (Knoxville, Tennessee) for his valuable suggestions on the manuscript, and, together with both Steve Cairns (Smithsonian Institution, Washington, DC, USA) and Bernard Lathuilière (Nancy, France), for many discussions on coral taxonomy. We are very grateful to two anonymous reviewers for their most helpful comments. Type material and additional study material was made accessible to us by Walter Etter (Naturhistorisches Museum Basel, Switzerland); Georg Friebe (“Inatura”, Dornbirn, Austria); Heinz Furrer (University of Zurich, Switzerland); Andreas Kroh, Alexander Lukeneder, Oleg Mandic, and Thomas Nichterl (all Naturhistorisches Museum, Vienna, Austria); Hans Egger (Geologische Bundesanstalt, Wien; GBA, Vienna, Austria); Sylvain Charbonnier and Christine Perrin (both Museum d’Histoire Naturelle de Paris, France); Helena Eliášová (Prague, Czech Republic); Elžbieta Morycowa (University of Krakow, Poland); Ewa Roniewicz (Academy of Sciences, Warsaw, Poland); Winfried Werner and Martin Nose (both Bayerische Staatssammlung, Munich, Germany); Günter Schweigert (Staatliches Museum für Naturkunde, Stuttgart, Germany); Jill Darrell (The Natural History Museum, London, UK); Ursula Menkveld-Gfeller (Naturhistorisches Museum Bern, Switzerland); Dieter Korn (Naturhistorisches Museum Berlin, Germany). Special thanks go to Robin Näf (Wildhaus) who generously provided us with material that proved to be very valuable to our study. As a Research Associate of the Smithsonian Institution (SI) Washington, DC, USA, and an Honorary Researcher at the Research Institute Senckenberg, Frankfurt, Germany, the author RBS would like to express her deep appreciation for the continuing support from these institutions.
Funding
Open Access funding enabled and organized by Projekt DEAL. The authors received no funding by third parties and we have no competing interests.
Author information
Authors and Affiliations
Contributions
PK and KT discovered the specimens taxonomically described. KT prepared the material. RBS had the idea to describe the material. KT photographed the specimens. All authors contributed to the illustrations. RBS wrote most of the text. PK and KT wrote parts of the text on the origin of the material and sample location. All authors proofread, corrected, and approved of the entire text. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Editorial handling: Michael Hautmann.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
See Tables 5, 6, 7, 8 and Figs. 7, 8, 9 and 10.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Baron-Szabo, R.C., Tschanz, K. & Kürsteiner, P. Scleractinian corals from the Lower Cretaceous of the Alpstein area (Anthozoa; Vitznau Marl; lower Valanginian) and a preliminary comparison with contemporaneous coral assemblages. Swiss J Palaeontol 141, 3 (2022). https://doi.org/10.1186/s13358-021-00238-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13358-021-00238-8









